Abstract
Tobacco use is the leading cause of preventable deaths worldwide. This habit is not only debilitating to individual users but also to those around them (second-hand smoking). Nicotine is the main addictive component of tobacco products and is a moderate stimulant and a mild reinforcer. Importantly, besides its unconditional effects, nicotine also has conditioned stimulus effects that may contribute to the tenacity of the smoking habit. Because the neurobiological substrates underlying these processes are virtually unexplored, the present study investigated the functional involvement of the dorsomedial caudate putamen (dmCPu) in learning processes with nicotine as an interoceptive stimulus. Rats were trained using the discriminated goal-tracking task where nicotine injections (0.4 mg/kg; SC), on some days, were paired with intermittent (36 per session) sucrose deliveries; sucrose was not available on interspersed saline days. Pre-training excitotoxic or post-training transient lesions of anterior or posterior dmCPu were used to elucidate the role of these areas in acquisition or expression of associative learning with nicotine stimulus. Pre-training lesion of p-dmCPu inhibited acquisition while post-training lesions of p-dmCPu attenuated the expression of associative learning with the nicotine stimulus. On the other hand, post-training lesions of a-dmCPu evoked nicotine-like responding following saline treatment indicating the role of this area in disinhibition of learned motor behaviors. These results, for the first time, show functionally distinct involvement of a- and p-dmCPu in various stages of associative learning using nicotine stimulus and provide an initial account of neural plasticity underlying these learning processes.
Keywords: tobacco, smoking, dorso-medial CPu, dorso-medial striatum, learning, nicotine stimulus
1. Introduction
In the United States alone, tobacco consumption is responsible for a fifth of all deaths (480,000 deaths per year) and more than $300 billion a year in expenditures related to health care and productivity loss (USDHHS, 2014). Nicotine is the primary addictive component of tobacco and is a mild stimulant and a relatively weak reinforcer (Chaudhri et al., 2006; Palmatier et al., 2007; Perkins, 1999). Previous research has been instrumental in advancing our understanding of nicotine’s primary reinforcing and behavioral or psychological effects that include reward, analgesia, and psychomotor activation among many others (Balfour, 2004; Damaj et al., 1998; Markou, 2008). Although studying nicotine’s primary reinforcing properties and their behavioral and neurobiological effects is of great importance to understanding tobacco addiction, learning processes involving nicotine are likely to be more complex and there is a need to study this complexity.
Researchers are increasingly aware that certain forms of the associative learning, including both Pavlovian and instrumental conditioning, contribute to the tenacity of tobacco use and nicotine dependence (Bevins and Besheer, 2014). For example, nicotine’s pharmacological effects originating inside the body, and which comprise a complex multimodal internal or interoceptive stimulus, can come into association with such reinforcers as peer interaction, food, alcohol, and work breaks, to name a few. Hence, the interoceptive effects of nicotine can acquire some additional motivation or appetitive effects by association with other stimuli in the environment; such conditioning can exert a profound influence on behavior. This coupling of the interoceptive effects of nicotine with non-nicotine rewards co-occurring in the environment can be modeled in rodents. For example, when nicotine in a controlled manner is repeatedly paired with access to sucrose, it acquires the ability to evoke an anticipatory food-seeking response in rats (goal-tracking). Although there has been significant progress in understanding the behavioral aspects of learning with nicotine as an interoceptive stimulus (for review see Bevins and Murray, 2011), there remains a significant gap in understanding neural mechanisms underlying this type of learning (Charntikov et al., 2012).
We recently began elucidating the neurobiological loci involved in learning with the nicotine stimulus (Charntikov et al., 2012). This research used a discriminated goal-tracking (DGT) task where on some days rats received nicotine paired with access to sucrose; on separate interspersed saline days, sucrose was not available. Across sessions, nicotine comes to evoke a goal-tracking response in the form of increased snout entries into the receptacle where sucrose has been delivered in the past (Besheer et al., 2004; Murray and Bevins, 2007). Behaviorally, this learning follows many of the postulates of Pavlovian conditioning (Bevins and Murray, 2011; Murray et al., 2009) and likely simulates learning process in human smokers (Glautier et al., 1996). Using this model, we found that rats that had a reliable history of nicotine-sucrose association had significantly higher nicotine induced c-Fos expression in the dmCPu when compared to controls (Charntikov et al., 2012). Importantly, this effect was evident in the presence of two carefully designed conditions that served as controls. One control condition had equal exposure to nicotine and sucrose, but nicotine was not reliably paired with the sucrose presentation (only half of nicotine sessions paired with the sucrose). The second control condition had exposure to nicotine in a manner identical to the other two conditions; however, sucrose was never available for this subset of rats. Following training, rats in all conditions were challenged with either nicotine or saline and assessed in the absence of sucrose reward for their goal-tracking behavior and the immediate neuronal activation. Results of this preliminary study provide a first account of possible neurobiological loci involved in conditioning processes with interoceptive effects of drugs.
Although our previous report showed the involvement of dmCPu in learning with the nicotine stimulus (Charntikov et al., 2012), this correlational increase in c-Fos activity does not inform us about the functional involvement of dmCPu in these learning processes. In addition, because the anatomical connections within anterior-posterior axis of rat dorsal striatum are not homogeneous (Kelley et al., 1982) and can differ in their control of learning processes (Hikosaka et al., 1999; Jeanblanc et al., 2003; Yin et al., 2005; Murray et al., 2012), it is unclear whether anterior (a-dmCPu) or posterior (p-dmCPu) regions are differentially involved in learning with the nicotine stimulus. For example, Yin et al. (2005) showed that only lesion to p-dmCPu and not a-dmCPu disrupted instrumental learning during acquisition and expression learning phases (lever pressing for food reinforcer). On the other hand, Murray et al. (2012) showed that lesions to p-dmCPu disrupted instrumental learning (cocaine seeking) only during early learning stage while lesions to a-dmCPu and not p-dmCPu disrupted instrumental performance after extensive period of learning. Because our previous aforementioned study showed that the history of learning with nicotine as a conditioned stimulus for an appetitive reward, and not the nicotine or sucrose alone, evoked higher neural activation in dmCPu (Charntikov et al., 2012), the goal of this study was to systematically assess the role of a- and p-dmCPu in the acquisition or expression of learning with the nicotine stimulus. Based on previous reports we hypothesized that lesions to p-dmCPu would decrease acquisition of learning with nicotine stimulus while lesions to a-dmCPu would decrease the expression of learning with nicotine stimulus.
2. Materials and Methods
2.1. Animals
Subjects were experimentally naive male Sprague-Dawley rats (total n=79 purchased from Harlan Industries (275–290 g; Indianapolis, IN, USA). Rats were housed individually in a temperature- and humidity-controlled colony (12:12 light:dark cycle; lights on at 6 am). Water was freely available; access to chow (Harlan Teklad Rodent Diet; Harlan, Indianapolis, IN, USA) was restricted to maintain rats at 85% of their free-feeding body weight. This 85% target weight was increased by 2 g every four weeks from beginning of the study. The night before and for two days following surgery, food was freely available. Experimental protocols were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
2.2. Apparatus
Behavioral testing was conducted in commercially available chambers (ENV-008CT; Med Associates, Inc., St. Albans, VT, USA) enclosed in sound- and light-attenuating cubicles equipped with an exhaust fan. Each conditioning chamber had aluminum sidewalls, metal rod floors with polycarbonate front, back, and ceiling. A recessed receptacle (5.2 × 5.2 × 3.8 cm; l × w × d) was centered on one of the sidewalls. A dipper arm, when raised, provided access to 0.1 ml of 26% (w/v) sucrose solution in the receptacle. Access to the dipper was monitored by an infrared beam mounted 1.2 cm into the receptacle and 3 cm above the chamber floor. Beam breaks for dipper entries were monitored using Med Associates interface and software (Med-PC for Windows, version IV).
2.3. Drugs
Nicotine hydrogen tartrate, buprenorphine hydrochloride, and sodium pentobarbital (Sigma; St. Louis, MO, USA) were dissolved in 0.9% saline. NMDA and lidocaine hydrochloride (Sigma) were dissolved in sterile distilled water and pH was adjusted to 7.0 ± 0.2 with a dilute NaOH solution. Nicotine dose (0.4 mg/kg; reported as base) and the 5 min injection-to-placement interval was selected based on previous research (Charntikov et al, 2012).
2.4. Discriminated Goal-Tracking Task
Rats were subcutaneously (SC) injected with 0.4 mg/kg nicotine for three consecutive days before training to attenuate the initial locomotor suppressant effects of nicotine (Charntikov et al., 2012). For each daily training session, all rats were injected with either nicotine (0.4 mg/kg; SC) or saline 5 min before placement in the conditioning chamber for a 20-min session. During training, each rat received equal number of nicotine and saline sessions. Sessions were assigned using a unique pseudorandom order of nicotine and saline sessions for each rat with the condition that no more than two of the same session type occur in a row. On nicotine sessions, the interoceptive stimulus effects of nicotine were paired with intermittent access to sucrose. Access to sucrose was initiated between 124 to 152 s from the start of the session with 4 possible onset times randomized throughout the training phase. There were 36 separate 4-sec deliveries of sucrose per nicotine session. Time between sucrose deliveries ranged from 4 to 80 s. For interspersed saline sessions administered on separate days, sucrose was withheld. No cues were presented throughout the sessions and house light was off at all times.
2.5. Dependent Measures
For acquisition, dipper entry rate per second before the first sucrose delivery was used as our measure of learning (i.e., nicotine-evoked goal tracking). Using dipper entries before the first sucrose delivery avoids any influence of sucrose access on this measure of learning. Calculating rate of dipper entries was required given that time to the initial sucrose delivery varied across nicotine sessions. Dipper entries from an equivalent time at the start of the saline sessions was used to ensure a comparable rate measure in these baseline non-sucrose sessions. For testing, the total number of dipper entries across the 4-min test session was used as the dependent measure. Finally, latency to first dipper receptacle entry was used as a proxy to assess the effects of lesions on locomotor activity and motivation for sucrose reinforcement.
2.6. Experiment 1: The involvement of the anterior or posterior dmCPu in the acquisition of learning with the nicotine stimulus
2.6.1. Procedures
A 2 × 2 factorial design was used for this experiment with lesion (NMDA or vehicle) and region (a- or p-dmCPu) as between-subjects factors. Rats (N=44) were anesthetized with a mixture of ketamine (100 mg/kg; IM) and xylazine (10 mg/kg; IM) and placed in the stereotaxic apparatus (David Kopf Instruments, CA, USA). Two bilateral craniotomies were performed and NMDA (0.5 μl/side; 0.12 M [~ 17.65 mg/ml] concentration) or vehicle (distilled water) was injected into either anterior (A/P +1.2, M/L ±1.6, D/V +4.2) or posterior (A/P −0.36, M/L ±2.4, D/V +4.2) dmCPu (see Figure 1; coordinates from Paxinos and Watson, 2007). Injections were made using a 28 gauge cannula (Plastics One, Roanoke, VA, USA) attached via tubing to a Hamilton microsyringe (10 μl; Reno, NV, USA) mounted on a single infusion pump (Fisher Scientific; Pittsburg, PA, USA). Infusions were made at a constant rate of 0.1 μl/min and cannula was left in place for an additional 5 min. Anesthesia was terminated using IM injection of 0.5 mg/kg atipamezole diluted in saline (Charntikov et al., 2013; Wee et al., 2006). Buprenorphine hydrochloride (0.1 mg/kg; SC) was injected immediately following surgery and the next day (am and pm) for pain management. Seven days after permanent inactivation of a- or p-dmCPu, rats were trained for 20 days (10 nicotine and 10 saline sessions) using the DGT task described above.
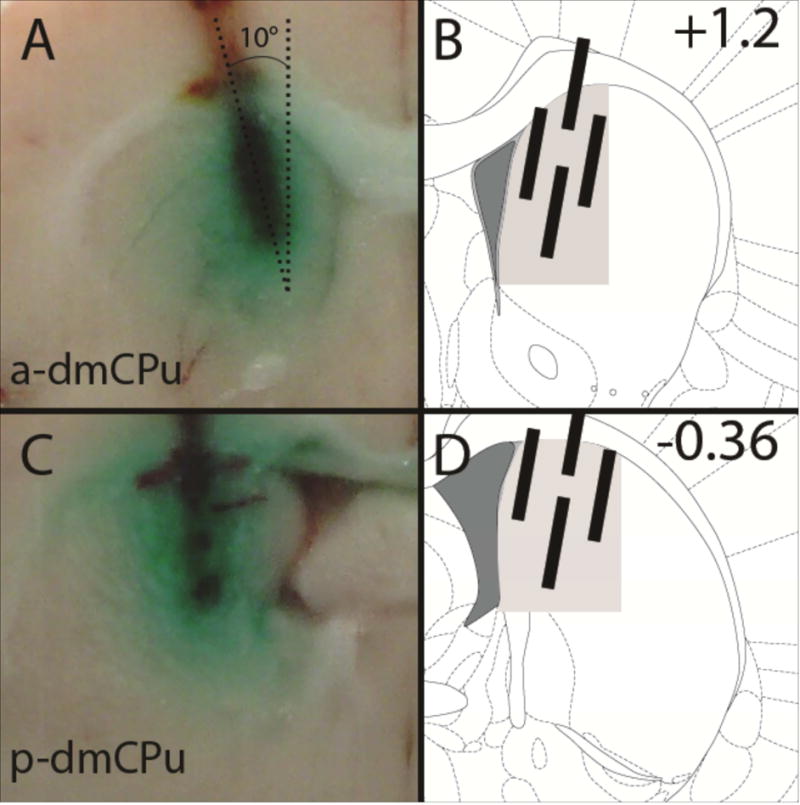
Figure 1.

Photomicrographs of the representative NeuN stained (A) a-dmCPu and (C) p-dmCPu NMDA lesions. (B and D) Graphical illustration of the extent of lesions; black area represents largest extend of the damage and grey areas represent smaller lesion sites. Dashed lines on the left panels (A and C) trace the exact boundaries of lesion sites. Dashed lines on the right panels (B and D) trace the arbitrarily predetermined dorsomedial target area. Numbers on the right panels indicate targeted Bregma position.
2.6.2. Histology
The day after the last training session, all rats were overdosed with sodium pentobarbital (150 mg/kg) and transcardially perfused with 0.9% saline following by 4% paraformaldehyde. Brains were excised and processed for the NeuN immunoreactivity as previously described (Charntikov et al., 2012). Briefly, following perfusion, tissue was post-fixed (4% paraformaldehyde) for an additional 24 hrs and then cryoprotected in 30% sucrose for another 72 hrs. Immediately after, brains were flash-frozen on dry-ice and stored at −80°C until sectioning. Coronal sections (40 μM) were taken using cryostat microtome and stored for no more than 48 hrs in 0.02 M phosphate-buffered saline (PBS) containing 0.1% sodium azide. For NeuN immunohistochemistry, brain sections were blocked for 1 hr with 10% normal horse serum (NHS; Vector Laboratories, CA, USA), 1% bovine serum albumin (BSA), and 0.3% Triton X-100 in 0.02 M PBS before 30-min incubation in 1.5% hydrogen peroxide and 50% methanol solution. Sections were then washed three times for 10 min in a wash buffer (0.02 M PBS containing 0.05% NHS and 0.3% Triton X-100). Sections were then incubated for 48 hrs at +4°C with anti-NeuN monoclonal primary antibody (clone A60; 1:5000 dilution; EMD Millipore Chemicals, MA, USA) diluted in PBS containing 0.3% Triton X-100, 1% NHS, and 1% blocking reagent (Roche Diagnostics, Mannheim, Germany). Following primary immunoreaction, sections were rinsed in a wash buffer three times for 10 min and incubated for 2 hrs on ice with a biotinylated horse anti-mouse secondary antibody (1:200 dilution; Vector Laboratories, CA, USA) diluted in PBS containing 1% NHS. Sections were then rinsed with 0.02 M PBS and incubated for 1 hr on ice with horseradish peroxide avidin-biotin complex (1:200 dilution; Vectastain Elite ABC Kit, Vector Laboratories) diluted in 0.02 M PBS. Immunolabled proteins were visualized with the aid of diaminobenzidine-based peroxide substrate (DAB Peroxidase Substrate Kit, Vector Laboratories) and mounted on gelatin-coated slides. Slides were air dried at room temperature, dehydrated in alcohol, cleared in xylene, and cover-slipped with mounting medium (Permount; Fisher Scientific, Fair Lawn, NJ, USA). Images of stained lesioned areas were taken with a light microscope (Olympus CX41RF microscope, Japan; 4×) and assessed for cell loss.
2.6.3. Statistical Analysis
During acquisition training there was no behavioral differences between rats with sham lesions to either a- or p-dmCPu (no effect of Group and no Group × Session interaction). Accordingly, they were combined into one sham group. Thus, the 3 groups were: shams (n=17), a-dmCPu (n=13), and p-dmCPu (n=14). Omnibus mixed effects ANOVA preceded all planned statistical assessments. To assess whether lesions to a- or p-dmCPu significantly affected learning with the nicotine stimulus we assessed variance of a primary dependent measure (dipper entries) over nicotine or saline sessions using separate ANOVAs. Violation of Mauchly’s tests for Sphericity were followed by Greenhouse-Geisser Sphericity corrections. To assess the effect of lesions on latency to first dipper receptacle entry we performed additional ANOVAs where appropriate. Pairwise comparisons were analyzed using Fisher LSD tests. In addition to the traditional group analysis, we used a regression analysis to reveal the effect of individual lesion differences on the acquisition of the discrimination with the nicotine stimulus. This type of analysis allows a better understanding of the role of independent measures on the acquisition of discriminated learning. Because lesions typically vary slightly in their position on the anterior-posterior axis, we used individual Bregma position of each lesion independent of the group assignment as a single continuous variable to further investigate the nature of the effect. Using this approach, both a- and p-dmCPu groups were pooled together (n=27) and acquisition of goal-tracking responding (dipper entries) was assessed as a factor of a lesion placement on the anterior-posterior axis. Thus, difference in dipper entry rate prior to the first sucrose delivery, or equivalent time during no reward sessions, between each lesioned rat and a mean dipper entry rate of sham controls for each corresponding nicotine session was used as a dependent measure. The difference score was calculated for each lesioned rat. The effect of lesion placement on this difference score was analyzed by fitting a linear model (Bregma × Session) and examining the fit using F-statistics. ANOVA of regression table followed regression analysis to determine significant predictor.
2.7. Experiment 2: The involvement of the anterior or posterior dmCPu in the expression of learning with the nicotine stimulus
2.7.1. Procedures
A 2 × 2 × 2 mixed-subjects factorial design was used for this experiment with region (a- or p-dmCPu) as between-subjects factor, and inactivation infusion (lidocaine or saline) and test drug (nicotine or saline) as within-subjects factors. All rats (N=30) were initially trained for 28 days (14 nicotine and 14 saline sessions) in the DGT task. The procedures used in this phase were identical to training described in Experiment 1. Following the training phase, rats underwent a surgery where stainless steel single guide cannulae (22 gauge; Plastics One, Roanoke, VA, USA) were implanted 2 mm above the a- or p-dmCPu. Anesthesia, coordinates, and post-surgical care was administered as described in Experiment 1. After seven days of recovery, rats were retrained for 10 days (5 nicotine and 5 saline sessions). Transient inactivation tests occurred following this initial retraining with additional retraining sessions in between each additional test (see Figure 2 for experimental time-line). On the test day, rats received intracranial microinjections of either lidocaine or distilled water. Lidocane dose and its infusion volume for this experiment were selected from previously published studies to functionally block an area in size comparable to dmCPu (area of interest for this study) with high inactivation rate (>90% of neurons) within that area (Hiranita et al., 2006; Kantak and Nic Dhonnchadha, 2011; Sandkühler et al., 1987; Sandkühler and Gebhart, 1984; Tehovnik and Sommer, 1997). Lidocaine (100 μg/0.5 μl/side) or vehicle (distilled water/0.5 μl/side) were infused in a room distinct from the testing environment and especially equipped for this procedure. Stainless steel stylets were replaced by 28 gauge infusion cannulae (Plastics One) which extend 2 mm below the guide cannulae. Hamilton microsyringes (10 μl), attached to two single infusion pumps (Fisher Scientific, Pittsburg, PA, USA), bilaterally infused assigned solution over 3 min and were left in place for additional 2 min after infusion. Five minutes after intracranial microinjections, rats were subcutaneously injected with either nicotine or saline. Five minutes later, rats were placed in the conditioning chamber for a brief 4-min test during which dipper entries were recorded but sucrose was not available. Prior to Experiment 2, we conducted a small dye dispersion study (a-dmCPu n=3; p-dmCPu n=2) to estimate the appropriate infusion volume based on the extent of vehicle dispersion 5 min after the infusion. Examination of coronal sections indicated that dye dispersion within dorsomedial regions of CPu was within predetermined areas (see Figures 3A and C).
Figure 2.

Procedural progression and the time line for the Experiment 2.
Figure 3.
Photomicrographs of vehicle/dye dispersion in the (A) a-dmCPu or (C) p-dmCPu, 5 min following infusion. Dashed lines indicate 10° angle of cannulae placement for a- and p-dmCPu. (B and D) Graphical representation of targeted areas (shaded in gray) and the acceptable deviations of injector placement on the medial-lateral and ventral-dorsal axis. Numbers on the right panels indicate targeted Bregma position.
2.7.2. Histology
The day after the last test, all rats were overdosed with sodium pentobarbital (150 mg/kg) and tissue was prepared for histological assessment as described in Experiment 1. Brain sections with visible cannulae tracks were visualized with thionin and assessed for placement within the predetermined areas (see Figures 3B-D and 4).
Figure 4.
Reconstructed cannulae placement on the anterior-posterior axis from Experiment 2.
2.7.3. Statistical Analysis
Performance during DGT training and retraining phases of this experiment was assessed using repeated measures ANOVAs with Drug (nicotine or saline) and Session as within-subjects factors. The effect of transient dmCPu inactivation on expression of nicotine-evoked responding was investigated using separate ANOVAs for each lesion condition (a- or p-dmCPu) with inactivation infusion (lidocaine or saline) and test drug (nicotine or saline) as within-subjects factors. To assess the effect of lesions on locomotor activity and motivation for sucrose reinforcement we assessed variance of latency to reach sucrose receptacle using additional ANOVAs. Finally, because we observed much less variability in cannulae placement than in placement of excitotoxic lesions on the anterior-posterior axis (Figure 4), we did not assess individual performance as a factor of cannulae placement on the Bregma scale. This reduced variability is likely because in Experiment 2 we used cannulae placement as our marker of lesion placement unlike the actual lesion sites in Experiment 1 which carry additional variance due to the individualized pattern of lesion infusate dispersion. Violations of assumptions of Sphericity and pairwise comparisons were handled as described in Experiment 1.
3. Results
3.1. Experiment 1
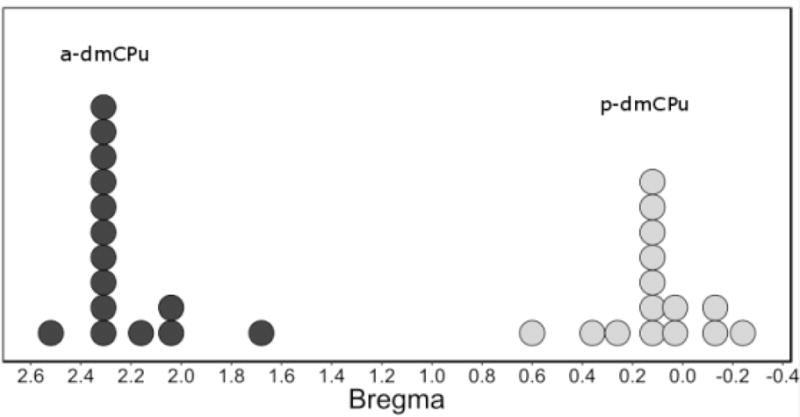
Figure 1 shows the typical extent of the lesion sites for the a-dmCPu (A) and the p-dmCPu (C) with observed variation in lesion size (B and D). Appropriate lesion placement was assessed by reconstructing NeuN stained lesions on the coronal atlas templates (Paxinos and Watson, 2007) and verifying that at least 75% of the lesion was localized to the predefined dorsomedial region. Individual lesion placement on the anterior-posterior axis, including misplaced lesions, is depicted in Figure 5.
Figure 5.
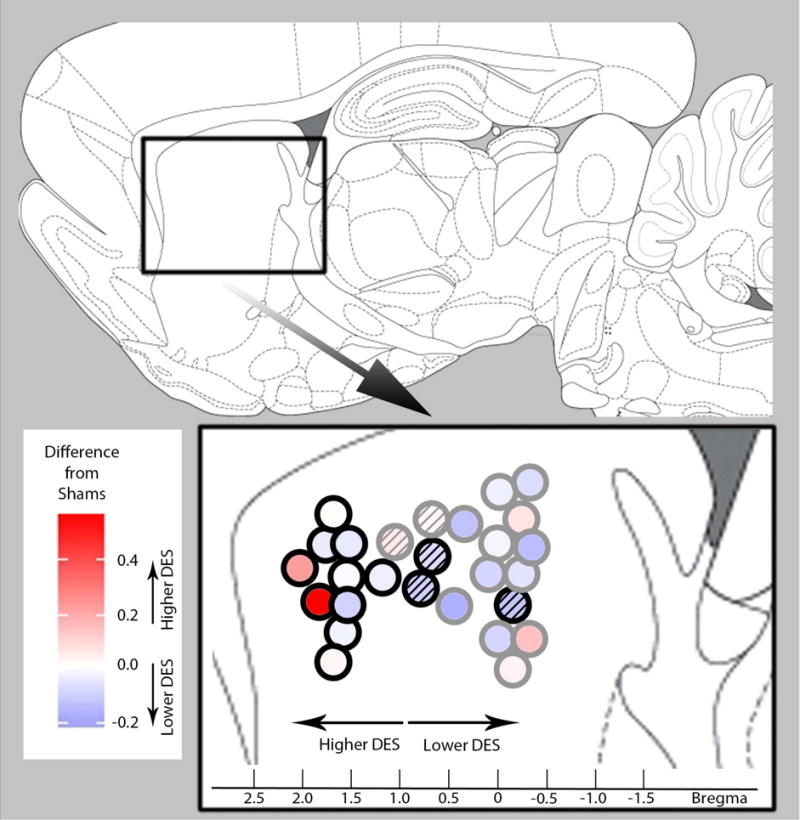
Heat map of the aggregated difference score for each lesioned rat. Each circle represents lesion placement on anterior-posterior axis (Bregma). Position of circles on a ventral-dorsal axis is not an accurate representation of lesion placement on the ventral-dorsal axis and is arranged in that way to better visualize each rat datum without obstruction. Black circles represent lesions from rats in a-dmCPu group and gray circles represent lesions from rats in p-dmCPu group. Fill color of the circles indicates the magnitude of an aggregated difference score (see methods for details) from sham controls. White color represent control-like responding, red hue represents higher than control responding, and blue hue represents lower than control responding (consult color scale on left). Circles shaded with 45° lines represent lesions from rats that were removed from the group analysis but not from regression analysis.
3.1.1. Group Effects
Lesions from 5 rats were outside of targeted areas and data from these subjects was excluded from group analysis. The omnibus ANOVA on the dipper entry rates during acquisition of the discrimination revealed a main effect of Group [a- or p-dmCPu; F(2,36)=4.71, p<0.05], a main effect of Drug [nicotine or saline; F(1,36)=145.71, p<0.001], a main effect of Session [1–10; F(9,324)=22.13, p<0.001], and a significant Drug × Session [F(9,324)=38.45, p<0.001] interactions. A separate ANOVA of responding on nicotine sessions (analysis of nicotine acquisition curves) revealed a main effect of Group [F(2,36)=3.84, p<0.05] and a main effect of Session [F(9,324)=38.06, p<0.001]. There was no Group × Session interaction. Overall, responding of rats with lesions to p-dmCPu on nicotine sessions was lower than responding of sham controls (Figure 6A). In comparison, goal-tracking responding of rats with lesions to a-dmCPu on nicotine sessions did not differ from shams. Additional ANOVA of latency to dipper receptacle on nicotine sessions revealed no effect of Group [F(2,36)=1.49, p=0.24], a main effect of Session [F(9,324)=26.14, p<0.001], and no Group × Session interaction [F(18,324)=1.30, p=0.18; see Supplemental Figure 1A for visualization of latency measure].
Figure 6.
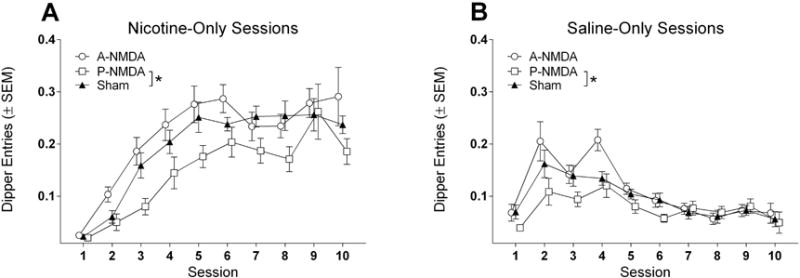
Nicotine (A) and saline (B) discrimination curves for groups of rats with NMDA lesions to a-dmCPu, p-dmCPu, and sham controls (Sham).
Analysis of responding on saline sessions (Figure 6B) revealed the effects of Group [F(2,36)=4.33, p<0.05], and Session [F(9,324)=14.94, p<0.001], but no significant interaction. Group mean comparisons revealed that goal-tracking responding of rats with lesions to p-dmCPu on saline sessions was overall lower than sham controls. Additional ANOVA of latency to dipper receptacle revealed no effect of Group [F(2,36)=0.96, p=0.39], a main effect of Session [F(9,324)=4.24, p<0.001], and significant Group × Session interaction [F(18,324)=2.11, p<0.01]. Within session comparisons to latencies of Sham controls revealed that latency of rats with lesions to p-dmCPu was higher than controls on sessions 1 and 2 (Figure 1B).
Overall, this outcome suggests that p-dmCPu and not a-dmCPu may be critically involved in the acquisition of interoceptive learning with the nicotine stimulus. However, to further confirm the critical role of p-dmCPu in acquisition of learning with the nicotine stimulus, we conducted an additional set of analyses which are presented below along with a rationale for the analytical approach.
3.1.2. Lesion Placement Effects
One of the inherent limitations associated with group analysis is the minimization or exclusion of often important individual differences. For example, the between-subjects or a group factor in Experiment 1 is the lesion placement (anterior vs. posterior dmCPu). Having lesion as a between-subjects factor relies on the confidence of lesion placement at the designated targets; with tighter group lesion clustering minimizing the error variance. However, this type of experimental design often produces a greater than anticipated distribution of the lesion placement on the anterior-posterior axis. The variance on the anterior-posterior axis is often greater than the variance on either lateral or ventral-dorsal axis because of a lack of definitive markers on the anterior-posterior scale (Bregma). Thus, this variation often contributes to the greater than expected spread of lesions on the anterior-posterior axis. Furthermore, because the diffusion of vehicle containing excitotoxin is not uniformed among the rats, this difference also contributes to the overall variance. To use this variation to our advantage in this study, we reconstructed each lesion placement on the Bregma scale and used the position on this scale as a continuous variable instead of a between-subjects factor (i.e., lesion group). The creation of such a continuous variable allowed us to conduct additional analyses and visualize individual behavioral differences as a factor of lesion placement on the anterior-posterior axis.
Regression analysis was used to test if the position of lesions on the anterior-posterior axis significantly predicted deviation of dipper entry rates from sham controls over the 10 training sessions. The results of regression indicated that Group and Session explained a significant proportion of variance in difference scores [R2=0.18, F(19,200)=2.42, p<0.01]. ANOVA of regression table revealed main effect of Bregma [F(1,200)=26.49, p<0.001], no effect of Session, and no Bregma × Session interaction. Simplifying the model, by removing non-significant factor (Session), revealed that lesion placement on the Bregma scale was a significant predictor of whether dipper entry rates would deviate from sham controls [β= 0.04, τ(218)=5.12, p<0.001]. We used heat map approach to effectively visualize combined data obtained from all lesioned rats (Figure 5). This way of assessment provides additional information otherwise unobtainable from assessment of group effects. For example, the heat map approach provides visual information regarding individual performance as a factor of lesion placement on the Bregma scale and possible spatial boundaries or transition points for the region-specific effects that we observed. With this approach, individual difference in responding on nicotine sessions in comparison to sham controls (difference scores) is plotted as colors and the variance is represented by the gradient value of single or multiple hues. With this in mind, Figure 5 shows aggregated difference score for each lesioned rat, represented as a blue-white-red color gradient, which is mapped horizontally on the Bregma scale of a sagittal atlas plate.
3.2. Experiment 2
Figures 3B and D show representation of the predefined dorsomedial region (shaded grey) and the acceptable cannula placement within its boundaries. All rats had cannulae placement in the predefined dorsomedial areas (see Figures 3 and 4).
3.2.1. DGT Training and Retraining
Over the 14 training sessions, rats rapidly acquired nicotine-evoked goal-tracking (Figure 7A). The analysis of dipper entry rates during this training phase revealed significant main effects of Drug [F(1,29)=156.26, p<0.001], Session [F(13,377)=10.70, p<0.001], and a significant Drug × Session interaction [F(13,377)=27.65, p<0.001]. Responding on nicotine sessions 1–2 was lower and on sessions 4–14 was higher than on corresponding saline sessions (Figure 7A). Following cannulae implantation surgery, rats in all conditions were sufficiently retrained for each inactivation test; that is, their responding on nicotine sessions prior to test days was higher than on a corresponding saline sessions (Supplemental Figure 2; see Supplemental Table 1 for main effects and interaction summaries).
Figure 7.
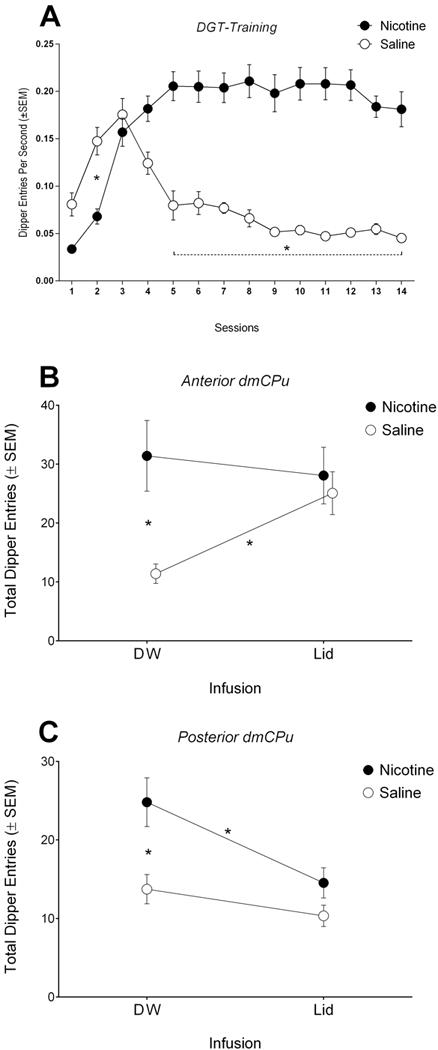
Dipper entry rates (±SEM) during initial training phase (A) and a mean (±SEM) number of total dipper entries during nicotine or saline 4-min test following either distilled water (DW) or lidocaine (Lid) infusion into (A) a-dmCPu or (B) p-dmCPu. *Denotes significant differences between corresponding data points.
3.2.2. a-dmCPu condition: Transient Inactivation
Data from all 4 tests were combined into one dataset for this analysis. There was significant main effect of Drug [F(1,14)=8.40, p<0.5], no effect of Infusion [F(1,14)=2.87, p=0.11], and a significant Drug × Infusion interaction [F(1,14)=6.00, p<0.05]. Following distilled water infusion (control), nicotine-evoked responding was higher than responding after saline injections; there was no effect of cannula implantation or vehicle infusion on the expression of discrimination performance (Figure 7B; see distilled water (DW) infusion). Lidocaine induced inactivation of a-dmCPu resulted in responding that was comparable to that typically controlled by nicotine; dipper entries following saline injection were higher after a-dmCPu inactivation (Figure 7B; see lidocane (Lid) infusion). On the other hand, lidocaine induced inactivation of a-dmCPu did not affect responding when tested with nicotine. Finally, additional analysis of latency to dipper receptacle revealed no effect of Drug [F(1,14)=0.45, p=0.51], no effect of Infusion [F(1,14)=0.07, p=0.78], and no Drug × Infusion interaction [F(1,14)=0.02, p=0.87].
3.2.3. p-dmCPu condition: Transient Inactivation
Data for this analysis were aggregated from 4 separate inactivation tests. There were significant main effects of Drug [F(1,14)=10.07, p<0.01] and Infusion [F(1,14)=10.88, p<0.01], as well as significant Drug × Infusion interaction [F(1,14)=5.03, p<0.05]. There was no effect of cannula implantation or vehicle infusion on the expression of discrimination performance as nicotine-evoked responding was higher than responding after saline injections following distilled water (control) infusions into p-dmCPu (Figure 7C; see distilled water (DW) infusion). Lidocaine infusions into p-dmCPu attenuated nicotine-evoked responding (compare nicotine responding following distilled water or lidocaine infusions; Figure 7C). Responding on nicotine test following lidocaine infusion was not statistically different from responding on saline test following lidocaine infusion (Figure 7C; see lidocane (Lid) infusion). Additional analysis of latency to dipper receptacle revealed no effect of Drug [F(1,14)=0.99, p=0.34], no effect of Infusion [F(1,14)=1.03, p=0.32], and no Drug × Infusion interaction [F(1,14)=0.99, p=0.33].
4. Discussion
We recently reported that the dmCPu was likely involved in associative learning with nicotine as a pharmacological interoceptive stimulus. That is, after a history of nicotine-sucrose pairings, nicotine alone evoked a goal-tracking response which was associated with elevated c-Fos activity in the dmCPu (Charntikov et al., 2012). Because elevated expression of sub-cellular c-Fos protein provides only a correlational account of regional involvement in the behavior of interest, we followed this line of research in the present study by investigating the functional involvement of a- or p-dmCPu in acquisition and expression of associative learning with the nicotine stimulus.
Results of Experiment 1 confirmed the importance of dmCPu for the acquisition of associative learning with the nicotine stimulus. Pretraining lesions of the p-dmCPu attenuated acquisition of nicotine-evoked goal-tracking. Although all groups were eventually able to discriminate nicotine from saline injections, responding of rats with lesions to p-dmCPu was overall significantly lower on both nicotine and saline sessions. Importantly, this effect is unlikely driven by the impaired locomotion because latencies to dipper receptacle were not significantly affected on nicotine sessions and were only increased on saline sessions 1 and 2. The data presented in Figure 6 indicate that the rats with lesions to p-dmCPu are capable of head entries into the dipper at a level similar to controls. Thus the tendency for fewer head entries could reflect impaired acquisition of the early session chamber-sucrose association, rather than non-specific motor impairment. This deficit in performance suggests that intact function of this region is required for optimal performance at this learning task. Previous reports corroborate the importance of p-dmCPu in the early stages of learning in rodents. For example, inactivation of p-dmCPu impaired acquisition of instrumental responding for food reward (Corbit et al., 2012; Yin et al., 2005) and cue-induced cocaine-seeking under second-order schedule of reinforcement (Murray et al., 2012). Furthermore, in a Pavlovian conditioning task, where rats learn an association between an exterocetpive auditory stimulus and a food reward, lesion to p-dmCPu impaired acquisition of stimulus-outcome association (Corbit and Janak, 2010). These studies provide a converging evidence suggesting that p-dmCPu may be involved in a broad range of associative learning processes including, as demonstrated by our study, learning involving a polymodal pharmacological stimulus like nicotine.
Experiment 2 found differential involvement of the anterior and posterior sub-regions of dmCPu in the expression of nicotine-evoked responding. Interestingly, the reversible inactivation of a-dmCPu evoked nicotine-like responding on a saline test. Because CPu is a major inhibitory structure, with efferent GABAergic projections to Globus Pallidus par externa (GPe; the indirect pathway), Globus Pallidus par interna, and Substantia Nigra pars reticulata complex (GPi/SNr; the direct pathway), it seems that inactivation of a-dmCPu disinhibited responding that otherwise was controlled (evoked) by the nicotine stimulus. In contrast, inactivation of p-dmCPu attenuated established nicotine-evoked responding which parallels the results of Yin et al., (2005) study. In that study, inactivation of the p-dmCPu reduced rats sensitivity to the devaluation or degradation of reward following a period of instrumental learning with food. Therefore, our data pattern suggests a functional dissociation of well-established responding to nicotine stimulus where a-dmCPu is involved in inhibition of responding in the absence of nicotine stimulus, whereas the p-dmCPu is involved in activation of goal-tracking behavior when nicotine stimulus is detected.
One of the most interesting finds of our study is what we characterize as the context-induced disinhibition of established goal-tracking responding following transient a-dmCPu inactivation. Recall that on the test day, following lidocaine infusion into the a-dmCPu, rats showed elevated goal-tracking response following saline treatment. The level of responding on this saline test was comparable in the magnitude to that actually controlled by the nicotine stimulus. We interpret this effect as a context-induced disinhibition of the conditioned responding that otherwise would be inhibited with the intact functioning of the a-dmCPu. Recall the pattern seen in early acquisition of learning with the nicotine stimulus (Figure 7A). Over the first three sessions of discrimination learning, the responding increases on both saline and nicotine sessions. This increase in responding on early sessions indicates excitatory context-reward association because the test chamber is paired with sucrose 50% of the time. This early learning about the context and reinforcement is gradually inhibited (see decline in responding on saline sessions 4–9; Figure 7A) as nicotine provides superior information about reinforcement availability; nicotine is paired with sucrose 100% of the time. Based on these observations, we would like to speculate that on the later sessions of DGT task (4–14; Figure 7A), when nicotine is not detected, the context-evoked responding is likely inhibited by the a-dmCPu. On the other hand, after a period of learning, the goal-tracking response is likely disinhibited by the a-dmCPu when nicotine stimulus is detected.
Caudate-putamen plays an important role in initiation of goal-directed behaviors. The inhibitory neurons projecting directly from CPu to the pallidum form a direct pathway, while other caudal neurons inhibit GPe which connects to pallidum via the subthalamic nucleus and thus forming an indirect pathway (for review see Grillner et al., 2005; Nambu, 2008). Our results suggest that the expression of nicotine controlled goal-tracking response is governed by the delicate balance in activity of the direct and indirect pathways. Lesions to p-dmCPu inhibited the expression of nicotine evoked goal-tracking suggesting inactivation of the direct pathway. Activation of the direct pathway, which otherwise is tonically inactive, is needed to disinhibit thalamus and thus disinhibit goal-tracking response. Because the indirect pathway is tonically active and provides inhibition of learned motor responses, it is not plausible that inactivating its efferent projections can facilitate this effect. On the other hand, our findings suggest that transient lesions to a-dmCPu inhibited activity of the neurons forming the indirect pathway which manifested itself in disinhibition of context induced goal-tracking responding. Removing the inhibition from GPe, through transient lesion of the a-dmCPu, renders the “braking” mechanism impaired, or disinhibits motor areas (thalamus and superior colliculus). Because the goal-tracking was observed without nicotine administration, and thus without nicotine’s interoceptive stimulus effects, some other stimulus seems to be involved in the activation of this goal-tracking response. The most plausible candidate, in the absence of nicotine stimulus, is the chamber itself, which was paired with sucrose 50% of the time and controlled some goal-tracking in the beginning of training phase (Figure 7A, sessions 1–3). Therefore, the outcomes of our study suggest that a) a- and p-dmCPu are differentially involved acquisition and expression of associative learning with the nicotine stimulus, and b) the expression of nicotine evoked goal-tracking responding is likely to be reliant on the balance in activity of both the direct and the indirect pathways.
Supplementary Material
Acknowledgments
This work was supported by NIH research grant DA034389 and NIH Pre-Doctoral Fellowship DA034449.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors declare no conflict of interest.
References
- Balfour DJK. The neurobiology of tobacco dependence: a preclinical perspective on the role of the dopamine projections to the nucleus accumbens [corrected] Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2004;6:899–912. doi: 10.1080/14622200412331324965. [DOI] [PubMed] [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: A Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology. 2004;172:108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Interoception and learning: Import to understanding and treating diseases and psychopathologies. 2014 doi: 10.1021/cn5001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Murray JE. Internal Stimuli Generated by Abused Substances: Role of Pavlovian Conditioning and Its Implications for Drug Addiction. In: Schachtman TR, Reilly S, editors. Associative Learning and Conditioning Theory: Human and Non-Human Applications. Oxford University Press; New York, NY: 2011. pp. 270–289. [Google Scholar]
- Charntikov S, Swalve N, Pittenger S, Fink K, Schepers S, Hadlock GC, Fleckenstein AE, Hu G, Li M, Bevins RA. Iptakalim attenuates self-administration and acquired goal-tracking behavior controlled by nicotine. Neuropharmacology. 2013;75:138–144. doi: 10.1016/j.neuropharm.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Tracy ME, Zhao C, Li M, Bevins RA. Conditioned response evoked by nicotine conditioned stimulus preferentially induces c-Fos expression in medial regions of caudate-putamen. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:876–84. doi: 10.1038/npp.2011.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology. 2006;189:27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Janak PH. Posterior dorsomedial striatum is critical for both selective instrumental and Pavlovian reward learning. European Journal of Neuroscience. 2010;31:1312–1321. doi: 10.1111/j.1460-9568.2010.07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual alcohol seeking: Time course and the contribution of subregions of the dorsal striatum. Biological Psychiatry. 2012;72:389–395. doi: 10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Fei-Yin M, Dukat M, Glassco W, Glennon Ra, Martin BR. Antinociceptive responses to nicotinic acetylcholine receptor ligands after systemic and intrathecal administration in mice. The Journal of pharmacology and experimental therapeutics. 1998;284:1058–1065. [PubMed] [Google Scholar]
- Glautier S, Clements K, White JA, Taylor C, Stolerman IP. Alcohol and the reward value of cigarette smoking. Behavioural Pharmacology. 1996;7:144–154. [PubMed] [Google Scholar]
- Grillner S, Hellgren J, Ménard A, Saitoh K, Wikström MA. Mechanisms for selection of basic motor programs–roles for the striatum and pallidum. Trends in neurosciences. 2005;28:364–70. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakai K, Lu X, Nakahara H, Rand MK, Nakamura K, Miyachi S, Doya K. Parallel neural networks for learning sequential procedures. Trends in Neurosciences. 1999;22:464–471. doi: 10.1016/s0166-2236(99)01439-3. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8523–7. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, Hoeltzel A, Louilot A. Differential involvement of dopamine in the anterior and posterior parts of the dorsal striatum in latent inhibition. Neuroscience. 2003;118:233–241. doi: 10.1016/s0306-4522(02)00823-0. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Nic Dhonnchadha BÁ. Pharmacological enhancement of drug cue extinction learning: Translational challenges. Annals of the New York Academy of Sciences. 2011;1216:122–137. doi: 10.1111/j.1749-6632.2010.05899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJH. The amygdalostriatal projection in the rat-an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Markou A. Review. Neurobiology of nicotine dependence. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2008;363:3159–68. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Belin D, Everitt BJ. Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology. 2012;37:2456–2466. doi: 10.1038/npp.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. European journal of pharmacology. 2007;561:91–104. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Penrod RD, Bevins RA. Nicotine-evoked conditioned responding is dependent on concentration of sucrose unconditioned stimulus. Behavioural Processes. 2009;81:136–139. doi: 10.1016/j.beproc.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A. Seven problems on the basal ganglia. Current Opinion in Neurobiology. 2008;18:595–604. doi: 10.1016/j.conb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, Donny EC, Sved AF. The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug and Alcohol Dependence. 2007;89:52–59. doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates Sixth Edition. Elsevier Academic Press. 2007;170:547–612. [Google Scholar]
- Perkins KA. Nicotine self-administration. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 1999;1:1999. doi: 10.1080/14622299050011951. [DOI] [PubMed] [Google Scholar]
- Sandkühler J, Gebhart G. Relative contributions of the nucleus raphe magnus and adjacent medullary reticular formation to the inhibition by stimulation in the periaqueductal gray of a spinal nociceptive reflex in the pentobarbital-anesthetized rat. Brain Research. 1984;305:77–87. doi: 10.1016/0006-8993(84)91121-1. [DOI] [PubMed] [Google Scholar]
- Sandkühler J, Maisch B, Zimmermann M. The use of local anaesthetic microinjections to identify central pathways: a quantitative evaluation of the time course and extent of the neuronal block. Experimental Brain Research. 1987;68:168–178. doi: 10.1007/BF00255242. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA. Effective spread and timecourse of neural inactivation caused by lidocaine injection in monkey cerebral cortex. Journal of Neuroscience Methods. 1997;74:17–26. doi: 10.1016/s0165-0270(97)02229-2. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. The Health Consequences of Smoking50 Years of Progress A Report of the Surgeon General. 2014. [Google Scholar]
- Wee S, Wang Z, He R, Zhou J, Kozikowski AP, Woolverton WL. Role of the increased noradrenergic neurotransmission in drug self-administration. Drug and Alcohol Dependence. 2006;82:151–157. doi: 10.1016/j.drugalcdep.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. European Journal of Neuroscience. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


