Abstract
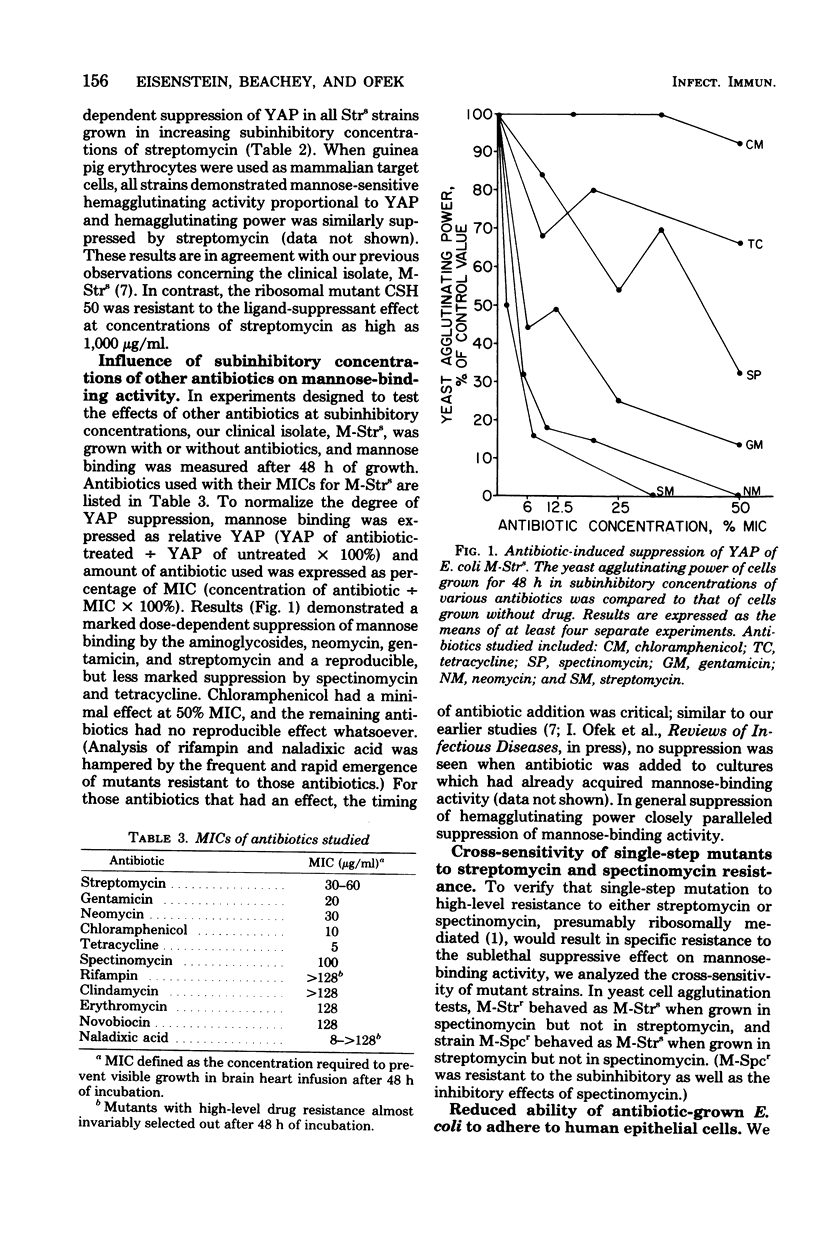
The ability of streptomycin, in subinhibitory concentrations, to differentially suppress the acquisition of the mannose-binding activity of Escherichia coli was demonstrated in several strains, but not one with a ribosomal mutation to high-level streptomycin resistance, rpsL. We also determined that the growth of bacteria in other antibiotics, notably those that interfere with protein synthesis, resulted in diminished mannose-binding activity (as measured by yeast cell agglutination), degree of piliation (as measured by electron microscopy), and adherence to human oral epithelial cells. The aminoglycoside antibiotics streptomycin, gentamicin, and neomycin had the most marked effects relative to their minimum inhibitory concentrations, followed by tetracycline. Both spectinomycin and chloramphenicol had more effect on adherence than on piliation, although spectinomycin had a more pronounced effect on mannose-binding activity than did chloramphenicol. We conclude that antibiotics, at concentrations below their minimum inhibitory concentration, may have profound effects on surface properties of bacteria that may be pertinent for their ability to colonize and infect human mucosal surfaces. The mechanism(s) may vary from one drug to another, but appear to depend on the classic actions of the antibiotics on inhibiting protein synthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Bermingham M. A., Deol B. S., Still J. L. Effect of streptomycin on lipid composition with particular reference to cyclic depsipeptide biosynthesis in Serratia marcescens and other micro-organisms. Biochem J. 1970 Oct;119(5):861–869. doi: 10.1042/bj1190861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G. D., Sox T., Blackman E., Sparling P. F. Factors affecting genetic transformation of Neisseria gonorrhoeae. J Bacteriol. 1977 Feb;129(2):983–992. doi: 10.1128/jb.129.2.983-992.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Coats J. H., Roeser J. In vivo inhibition of R-factor transfer by clindamycin and its analogues. J Antimicrob Chemother. 1977 Nov;3 (Suppl 100):53–58. doi: 10.1093/jac/3.suppl_c.53. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Ofek I., Beachey E. H. Interference with the mannose binding and epithelial cell adherence of Escherichia coli by sublethal concentrations of streptomycin. J Clin Invest. 1979 Jun;63(6):1219–1228. doi: 10.1172/JCI109417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima A., Childs G., Inouye M. Differential inhibitory effects of antibiotics on the biosynthesis of envelope proteins of Escherichia coli. J Mol Biol. 1973 Sep 15;79(2):373–389. doi: 10.1016/0022-2836(73)90012-0. [DOI] [PubMed] [Google Scholar]
- Jit Sud I., Feingold D. S. Detection of agents that alter the bacterial cell surface. Antimicrob Agents Chemother. 1975 Jul;8(1):34–37. doi: 10.1128/aac.8.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klainer A. S., Russell R. R. Effect of the inhibition of protein synthesis on the Escherichia coli cell envelope. Antimicrob Agents Chemother. 1974 Aug;6(2):216–224. doi: 10.1128/aac.6.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Yamagata H., Mizushima S. Interaction of cytoplasmic membrane and ribosomes in Escherichia coli: spectinomycin-induced disappearance of membrane protein I-19. J Bacteriol. 1977 Jan;129(1):326–332. doi: 10.1128/jb.129.1.326-332.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny C., Carnahan J., Brinton C. C., Jr Mechanical removal of F pili, type I pili, and flagella from Hfr and RTF donor cells and the kinetics of their reappearance. J Bacteriol. 1969 Jun;98(3):1294–1306. doi: 10.1128/jb.98.3.1294-1306.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]


