Abstract
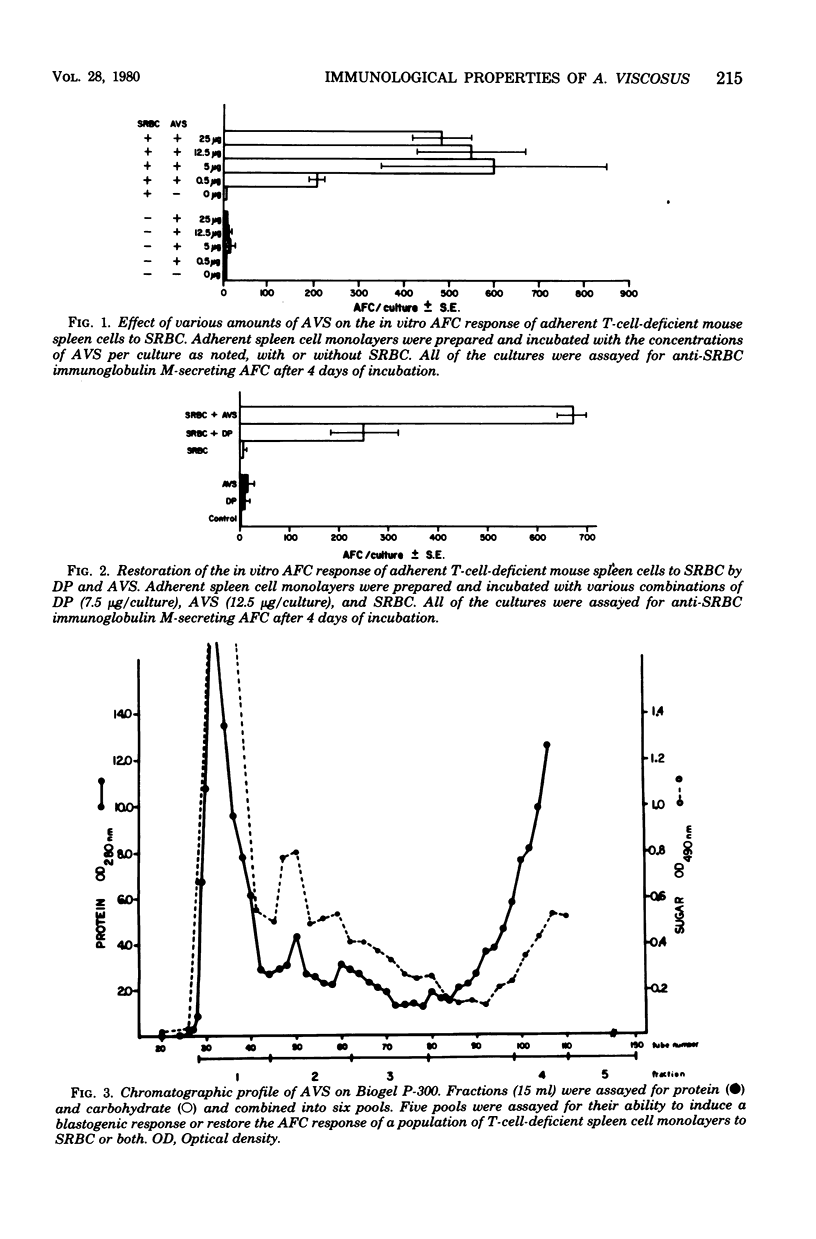
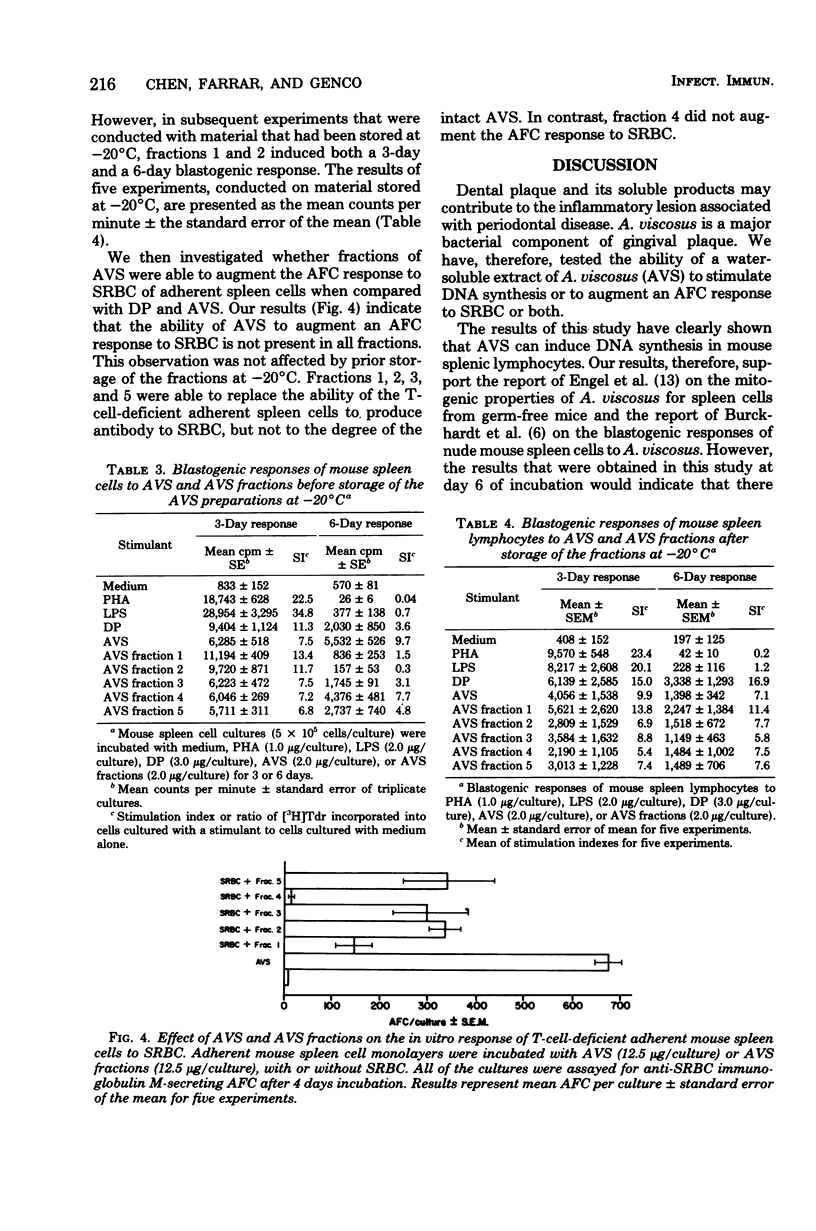
A water-soluble extract of Actinomyces viscosus was tested for its capacity to induce deoxyribonucleic acid synthesis in spleen cells from normal mice and to augment the in vitro antibody-forming cell (AFC) response to the T-cell-dependent antigen, sheep erythrocytes (SRBC) by T-cell-deficient adherent spleen cell monolayers from the same mice. Our results indicated that the water-soluble extract induced an in vitro blastogenic response, as assessed by tritiated thymidine incorporation into mouse spleen cells. The water-soluble extract also augmented the antibody response to SRBC. However, after fractionation of the water-soluble extract on Biogel P-300, not all of the fractions induced a blastogenic response and augmented an AFC response to SRBC. This difference in the ability of A. viscosus fractions to induce in vitro responses suggests that the water-soluble extract has multiple immunological properties. Chemical analysis of the water-soluble extract indicated an enrichment for amino acids and amino sugar common to peptidoglycan.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Davies A. J. Requirement of thymus-dependent lymphocytes for potentiation by adjuvants of antibody formation. Nature. 1971 Oct 1;233(5318):330–332. doi: 10.1038/233330a0. [DOI] [PubMed] [Google Scholar]
- Armerding D., Katz D. H. Activation of T and B lymphocytes in vitro. I. Regulatory influence of bacterial lipopolysaccharide (LPS) on specific T-cell helper function. J Exp Med. 1974 Jan 1;139(1):24–43. doi: 10.1084/jem.139.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audibert F., Chédid L., Lefrancier P., Choay J. Distinctive adjuvanticity of synthetic analogs of mycobacterial water-soluble components. Cell Immunol. 1976 Feb;21(2):243–249. doi: 10.1016/0008-8749(76)90053-8. [DOI] [PubMed] [Google Scholar]
- Baker J. J., Chan S. P., Socransky S. S., Oppenheim J. J., Mergenhagen S. E. Importance of Actinomyces and certain gram-negative anaerobic organisms in the transformation of lymphocytes from patients with periodontal disease. Infect Immun. 1976 May;13(5):1363–1368. doi: 10.1128/iai.13.5.1363-1368.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt J. J., Guggenheim B., Hefti A. Are Actinomyces viscosus antigens B cell mitogens? J Immunol. 1977 Apr;118(4):1460–1465. [PubMed] [Google Scholar]
- Burckhardt J. J. Rat memory T lymphocytes: in vitro proliferation induced by antigens of Actinomyces viscosus. Scand J Immunol. 1978;7(2):167–172. doi: 10.1111/j.1365-3083.1978.tb00440.x. [DOI] [PubMed] [Google Scholar]
- Campbell P. A., Schuffler C., Rodriguez G. E. Listeria cell wall fraction: a B cell adjuvant. J Immunol. 1976 Mar;116(3):590–594. [PubMed] [Google Scholar]
- Chen P., Farrar J. J., Oppenheim J. J., Mergenhagen S. E. Mechanism of adjuvant activity of dental plaque: in vitro activation of residual helper T-cell precursors in T-cell-deficient murine spleen cell cultures. Infect Immun. 1977 Sep;17(3):567–571. doi: 10.1128/iai.17.3.567-571.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Rodriguez G. E., Kind P. D., Campbell P. A. Listeria cell wall fraction: a B cell mitogen. J Immunol. 1975 Mar;114(3):1132–1134. [PubMed] [Google Scholar]
- Damais C., Parant M., Chedid L. Nonspecific activation of murine spleen cells in vitro by a synthetic immunoadjuvant (N-acetyl-muramyl-L-alanyl-D-isoglutamine). Cell Immunol. 1977 Nov;34(1):49–56. doi: 10.1016/0008-8749(77)90228-3. [DOI] [PubMed] [Google Scholar]
- Engel D., Clagett J., Page R., Williams B. Mitogenic activity of Actinomyces viscosus. I. Effects on murine B and T lymphocytes, and partial characterization. J Immunol. 1977 Apr;118(4):1466–1471. [PubMed] [Google Scholar]
- Genco R. J., Evans R. T., Ellison S. A. Dental research in microbiology with emphasis on periodontal disease. J Am Dent Assoc. 1969 May;78(5):1016–1036. doi: 10.14219/jada.archive.1969.0162. [DOI] [PubMed] [Google Scholar]
- Hoffmann M. K., Galanos C., Koenig S., Oettgen H. F. B-cell activation by lipopolysaccharide. Distinct pathways for induction of mitosis and antibody production. J Exp Med. 1977 Dec 1;146(6):1640–1647. doi: 10.1084/jem.146.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- JORDAN H. V., KEYES P. H. AEROBIC, GRAM-POSITIVE, FILAMENTOUS BACTERIA AS ETIOLOGIC AGENTS OF EXPERIMENTAL PERIODONTAL DISEASE IN HAMSTERS. Arch Oral Biol. 1964 Jul-Aug;9:401–414. doi: 10.1016/0003-9969(64)90025-1. [DOI] [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Shimono T., Narita T., Kato K. Immunoadjuvant activities of cell walls, their water-soluble fractions and peptidoglycan subunits, prepared from various gram-positive bacteria, and of synthetic n-acetylmuramyl peptides. Z Immunitatsforsch Exp Klin Immunol. 1975 Jul;149(2-4):302–319. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loughman B. E., Farrar J. J., Nordin A. A. Presence of antibody-forming cell precursors in the mouse adherent spleen cell population. J Immunol. 1974 Jan;112(1):430–432. [PubMed] [Google Scholar]
- Mashimo P. A., Ellison S. A. Diffusate media for cultivation of oral anaerobic bacteria. Koku Eisei Gakkai Zasshi. 1972 Mar;22(1):38–44. doi: 10.5834/jdh.22.38. [DOI] [PubMed] [Google Scholar]
- McGhee J. R., Farrar J. J., Michalek S. M., Mergenhagen S. E., Rosenstreich D. L. Cellular requirements for lipopolysaccharide adjuvanticity. A role for both T lymphocytes and macrophages for in vitro responses to particulate antigens. J Exp Med. 1979 Apr 1;149(4):793–807. doi: 10.1084/jem.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. doi: 10.1126/science.158.3808.1573. [DOI] [PubMed] [Google Scholar]
- Newburger P. E., Hamaoka T., Katz D. H. Potentiation of helper T cell function in IgE antibody responses by bacterial lipolysaccharide (LPS). J Immunol. 1974 Sep;113(3):824–829. [PubMed] [Google Scholar]
- Patters M. R., Genco R. J., Reed M. J., Mashimo P. A. Blastogenic response of human lymphocytes to oral bacterial antigens: comparison of individuals with periodontal disease to normal and edentulous subjects. Infect Immun. 1976 Nov;14(5):1213–1220. doi: 10.1128/iai.14.5.1213-1220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotz P. H., Talal N., Asofsky R. Assignment of direct and facilitated hemolytic plaques in mice to specific immunoglobulin classes. J Immunol. 1968 Apr;100(4):744–751. [PubMed] [Google Scholar]
- Reed M. J. Chemical and antigenic properties of the cell wall of Actinomyces viscosus (Strain T6). J Dent Res. 1972 Sep-Oct;51(5):1193–1202. doi: 10.1177/00220345720510050501. [DOI] [PubMed] [Google Scholar]
- Rodriguez G. E., McClatchy J. K., Campbell P. A. Induction of Resistance by Listeria monocytogenes Cell Wall Fraction. Infect Immun. 1974 Nov;10(5):1163–1169. doi: 10.1128/iai.10.5.1163-1169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROMINGER J. L., PARK J. T., THOMPSON R. E. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J Biol Chem. 1959 Dec;234:3263–3268. [PubMed] [Google Scholar]
- Socransky S. S. Relationship of bacteria to the etiology of periodontal disease. J Dent Res. 1970 Mar-Apr;49(2):203–222. doi: 10.1177/00220345700490020401. [DOI] [PubMed] [Google Scholar]
- Stewart-Tull D. E., Shimono T., Kotani S., Kato M., Ogawa Y., Yamamura Y., Koga T., Pearson C. M. The adjuvant activity of a non-toxic, water-soluble glycopeptide present in large quantities in the culture filtrate of Mycobacterium tuberculosis strain DT. Immunology. 1975 Jul;29(1):1–15. [PMC free article] [PubMed] [Google Scholar]
- Strong D. M., Ahmed A. A., Thurman G. B., Sell K. W. In vitro stimulation of murine spleen cells using a microculture system and a multiple automated sample harvester. J Immunol Methods. 1973 Apr;2(3):279–291. doi: 10.1016/0022-1759(73)90054-9. [DOI] [PubMed] [Google Scholar]
- Wexler H., Oppenheim J. D. Isolation, characterization, and biological properties of an endotoxin-like material from the gram-positive organism Listeria monocytogenes. Infect Immun. 1979 Mar;23(3):845–857. doi: 10.1128/iai.23.3.845-857.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Broady K. W., Evans J. D., Knox K. W. New cellular and extracellular amphipathic antigen from Actinomyces viscosus NY1. Infect Immun. 1978 Nov;22(2):615–616. doi: 10.1128/iai.22.2.615-616.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. L., Pantalone R. M., Sherris J. C. Subgingival microflora and periodontitis. J Periodontal Res. 1976 Feb;11(1):1–18. doi: 10.1111/j.1600-0765.1976.tb00045.x. [DOI] [PubMed] [Google Scholar]


