Abstract
Kaposi Sarcoma (KS) is the most common neoplasm of people living with HIV today. In Sub-Saharan Africa KS is among the most common cancers in men, overall. Not only HIV+ individuals present with KS; any immune compromised person infected with Kaposi Sarcoma-associated herpesvirus (KSHV) or human herpesvirus 8 is at risk: the elderly, children in KSHV-endemic areas, and transplant recipients. KS diagnosis is based on detection of the viral protein LANA in the biopsy, but not all cases of KS are the same or will respond to the same therapy. Standard KS therapy has not changed in 20 years, but newer modalities are on the horizon and will be discussed.
1. Introduction
It is somewhat disheartening, but after 20 years of intense research the cell of origin of Kaposi Sarcoma (KS) remains elusive. KS displays broad clinicopathological variation depending on (i) location of KS lesions (lymph node, internal, or cutaneous), (ii) clinical stage (patch, plaque, nodular) and (iii) epidemiological classification. The latter includes classic KS in elder men, African (endemic) KS in younger African men and children from central Africa, iatrogenic KS (mainly in transplant patients, but also due to chemotherapy and other immune suppressive therapy) and epidemic HIV / AIDS-associated KS [1]. Endemic KS includes a nodular clinically indolent form, an aggressive variant with large invasive cutaneous tumors with involvement of underlying soft tissue and bone, and an endemic-pediatric variant that presents as lymphadenopathy in young children [2, 3]. The overwhelming majority of epidemic HIV-associated KS occurs in men who have sex with men, but women, children, intravenous drug addicts and recipients of organ transplants are also affected [4]. Additional clinicopathological sub-groups of epidemic KS comprise HIV-associated KS on combined antiretroviral therapy (cART), HIV-associated KS with no or failing cART, i.e. end-stage AIDS, HIV-associated pediatric KS, and KS associated with immune reconstitution inflammatory syndrome (IRIS) [5]. Current evidence indicates that pediatric KS, regardless of the epidemiologic variant, is different from adult KS with an increased risk for disseminated and progressive disease [6]. The wide clinicopathological spectrum of KS suggests that KS does not represent a single disease. Yet, they are all treated the same: surgery/cryotherapy for superficial skin lesions and liposomal doxorubicin (Doxil™) as first-line therapy for systemic and more extensive forms of KS [7, 8]. Recently, more treatment options have gained acceptance and newer targeted therapies show promise in clinical trials.
2. Kaposi Sarcoma-associated herpesvirus (KSHV)
All variants of KS are caused by Kaposi sarcoma-associated herpesvirus (KSHV) [9–12] [13]. KSHV comprises six major subtypes (A, B, C, D, E, and F). Genetic variability of ORF-K1 gene sequences correlates to 13 different variants of KSHV [14]. Limited evidence exists that certain KSHV subtypes may correlate with more aggressive disease or with particular epidemiological types of KS [15, 16].
The molecular biology of KSHV has recently been reviewed in detail [17]. For the purpose of this article it is important to recall that KSHV is a double-strand DNA virus, which encodes more than 80 proteins and an extensive set of micro RNAs. The virus is enveloped and uses a virus-encoded DNA-dependent DNA polymerase (orf9) for genome replication during the lytic phase. The viral polymerase is sensitive to the drugs ganciclovir and foscarnet, but not to acyclovir. Like other herpesviruses KSHV also encodes a large number of genes that help it to escapes immune destruction during primary infection. These relate to the inhibition of the interferon response, anti-apoptosis factors, autophagy inhibitors, and also oppose Natural Killer (NK) cell-mediated control of KSHV infection (reviewed in [18]).
KSHV like all herpesviruses establishes molecular latency, including in KS tumor cells. At any given time only a low percentage of KS tumor cells replicate the virus, while the majority of cells only express the viral latent genes and viral latent miRNAs [19–21]. This gene expression pattern suffices to maintain KS lesions. The viral latency associated nuclear antigen (LANA) alone is necessary and sufficient to maintain the viral episome in dividing infected cells [22] (Figure 1); however, there exists evidence that lytic replication is required for systemic persistence, oncogenesis, and disease progression [23]. Obviously, viral replication is required for transmission, independent of KS lesion status.
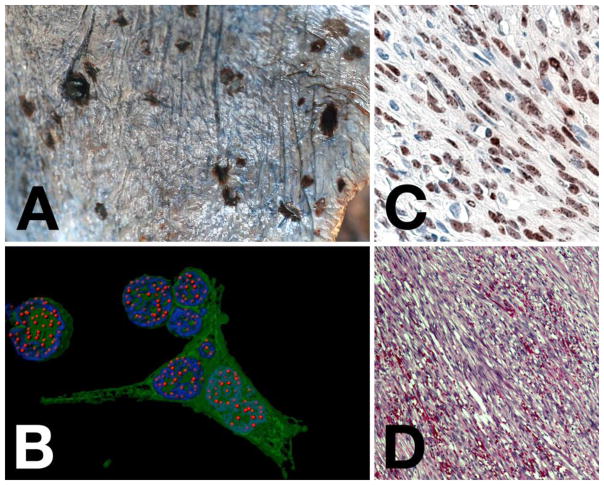
Figure 1. KS pathology and histology.
Panel A shows an image of gross morphology of disseminated KS on the surface of the lung. Note the single, raised, nodular lesion in the upper left, as compared to the flat lesions. Panel B shows a computer-enhanced image of immunofluorescence in a KSHV recombinant virus that also expressed green fluorescent protein (gfp) in a PEL cell line. LANA staining is in red, nuclear DNA staining in blue and gfp (to indicate infected cells) in green. This analysis clearly shows the presence of discrete “LANA dots”, each indicating a place where the viral genome is tethered to the host chromosome. Panel C shows an image of LANA staining of a KS lesion by immunohistochemistry (brown) with hematoxilin counterstain (blue). Note all LANA staining is nuclear and the appearance of darker spots or dots within the nuclear staining. Panel D, shows an H&E stain of a KS lesion. Note the spindle shape nature of the cells, which are of endothelial cell lineage. Slit-like spaces in between the cells contain extravasated red blood cells.
Transmission of KSHV is chiefly by horizontal transmission through saliva [24–28], though extensive, repeated contact is needed to establish transmission, such as it occurs in mother-to-child or sexual interactions. This scenario represents an important distinction to Epstein-Barr-Virus, which is present at significantly higher levels in saliva and readily transmitted, e.g. in college settings, where it leads to infectious mononucleosis. For adults heterosexual transmission of KSHV is not considered significant, but sexual transmission between MSM is important to explain the elevated population prevalence. KSHV seroconversion correlates with contact number [26]. Here too, saliva is considered to be a major factor. Transmission of KSHV though blood transfusions is rare, isolated cases of transmission by organ transplant have been reported [29], while vertical routes of transmission appear to be unimportant.
The fact that KS develops in HIV-negative transplant patients as well as in AIDS patients suggests that HIV in itself is not required for KS. The fact that the risk of developing KS is higher in KSHV seropositive HIV+ patients than in HIV− transplant patient, suggests that HIV infection may generate a qualitatively more deleterious immune deficiency than chemical or drug induced T cell inactivation. This notion is supported by results from the START trial [30]. Here, fewer patients developed KS if ART was started immediately at 500 CD4 cells/mm3 compared to deferred therapy initiation at still respectable 350 CD4 cells/mm3. Alternatively, HIV viral replication, HIV proteins, or HIV-associated immune activation may result in KSHV reactivation. On the one hand KSHV replication is known to precede KS tumorigenesis and this may explain the prophylactic efficacy of ganciclovir [23, 31], a KSHV polymerase inhibitor. On the other hand, KSHV replication is not required to maintain existing KS lesions, as these contain KSHV mostly in its latent form, which may explain why gancilovir had no effect on established KS lesions [32, 33].
T-cell specific immunosuppression is a well-recognized cofactor in late stage AIDS-KS and in transplant-associated KS, but less so in classic KS, HIV-negative endemic KS and in KS that develops in HIV-positive person on successful therapy, i.e. with near normal CD4 counts and undetectable HIV viral load. Whether persons that develop KS in the absence of massive CD4 cell deficiency suffer from more subtle impairments, such as anergy, immune senescence, loss of T cell repertoire [34], or whether there exist tumor triggers that operate entirely independently of immune surveillance is the subject of ongoing scientific inquiries.
3. Cell Lineage / origin and Differentiation in KS
The spindle-shaped cells in KS lesions are believed to be of endothelial lineage [35, 36], though they also have features of smooth muscles cells and pericytes [37–39]. There is even evidence that KS is a mixture of tumor cells. Even though spindle cells comprise the bulk of the lesion and the proliferating fraction, they may not be the driver population. Other stromal components, such as pericytes or even macrophages may be necessary to sustain the lesion and may secrete essential, lesion-driving paracrine factors [40]. If so, then targeting these cell populations, even though they are in a minority, may lead to advances in KS therapy. Finally, tumor stem cells in KS could perpetuate the tumor and spawn the highly proliferative spindle cells. Endothelial stem cells would be de-differentiated or trans-differentiated lineage specific endothelial cells (lymphatic endothelial cells (LEC) or blood-vessel endothelial cells (BEC)) [35–37, 41] that acquire traits of “stemness” (shared molecular pathways that underpin the fundamental stem cell properties of self-renewal and specific cellular differentiation), such as defined by loss of differentiation markers or gain of stemness markers, e.g. primitive mesenchymal cells.
4. Update on KS epidemiology – Classic, AIDS- and transplant KS
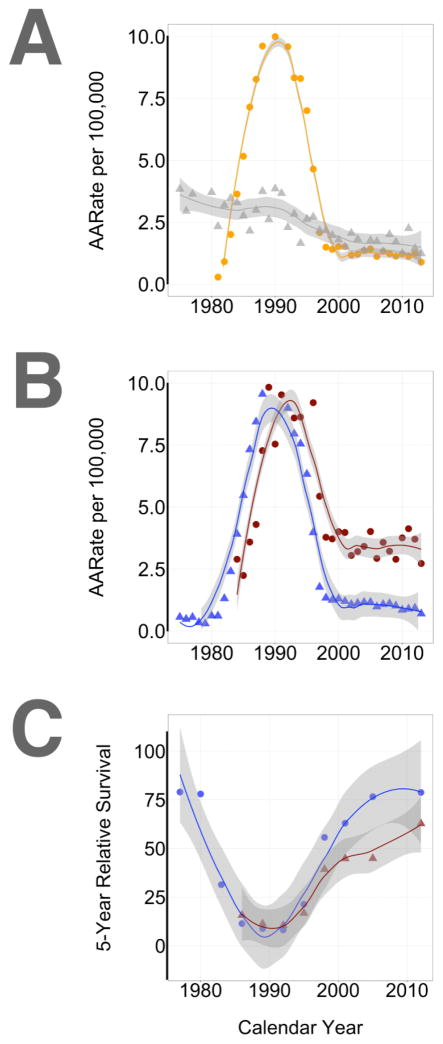
The epidemiology of KS in the US, i.e. an area of low KSHV and HIV seroprevalence and primarily adult transmission in high-risk groups, is driven by AIDS-KS between MSM and classic KS in the elderly. Figure 2 summarizes the most recent data as collected in the SEER database. Since only 6% of cases were female, only male cases are included. Firstly, KS incidence rates are stratified by age group (<65 and 65+) as a crude surrogate to separate classic and HIV-associated KS in a low KSHV seroprevalence region. Secondly, KS incidence rates are stratified by race. The peak of the US KS epidemic between 1981 and 1997 is clearly evident (Figure 2, panel A). Since 1997 the KS incidence has remained level at ~ 1/100,000. Today, we see as many cases developing in younger males as in older males. The incidence of AIDS-KS no longer declines after the year 2000 as both KSHV and HIV are now established in the US population. Dermatologists and oncologists will continue to see and treat KS lesions.
Figure 2. Review of KS incidence rates based on US SEER data (10-15-2016).
Data and graphs were obtained using the SEER data website (http://seer.cancer.gov/explorer/). For panels A–B the calendar year is shown on the horizontal and incidence rate per 100,000 on the vertical axis. Panel A stratifies data by age (orange circle: <65 and gray triangle: >65+). Panel B stratifies data by race/ethnicity (red circle: African American and White: blue triangle). Panel C shows 5-year survival by calendar interval and also stratifies data by race/ethnicity (red circle: African American and White: blue triangle).
These SEER data indicate a significant race/ethnicity disparity with respect to KS incidence and response to treatment. The HIV epidemic “hit” the black communities in the US somewhat delayed, but has continued at a higher level up to today [42]. This is reflected in a significantly higher rate of KS even in the most recent years (Figure 2, panel B). The 5-year survival for black KS patients is significantly worse than for white KS patients (Figure 2, panel C). All of the KS cases in blacks are in younger men (0–44 years old). In that age bracket the survival disparity continues to this day. In the 1990–1992 calendar period the 5-year survival for whites was 19.2% compared to 15.1% for black KS patients. In the most recent 2006–2012 calendar period the 5-year survival for whites was 75.3% compared to 59.4% for black KS patients. The reason for this discrepancy is unclear, but may point to a need for increased screening and education in addition to better treatment and overall health resources.
5. Update on KS epidemiology – Endemic, AIDS-KS
The epidemiology of KS in Sub-Saharan Africa and South Africa, i.e. an area of high KSHV and substantial HIV seroprevalence is driven by AIDS-KS and endemic KS in the elderly and children. Here, transmission is seen in the pediatric population (mother-to-child) as well as in the adult population and KSHV seroprevalence was substantial prior to the emergence of HIV. High quality incidence data are difficult to obtain, but WHO globocan [43, 44] and several other studies indicate that KS continues to comprise a significant percentage of total cancer burden in Sub-Saharan Africa, with 24% in Mozambique, 27% in Uganda and 35% in Zimbabwe [45–52]. The introduction of ART has significantly reduced the incidence of KS in HIV-infected patients, as eloquently illustrated by Bohlius et al. [53], who found that the early introduction of ART decreased the risk to develop KS by 80% in a cohort of HIV-infected South African patients. KS continues to increase in South African pediatric patients [54]. The HIV epidemic on the African continent is most severe in South Africa and in 2015 approximately 7 million South Africans lived with HIV. Despite the introduction of ART and actions to fight HIV infection, an estimated 380,000 new infections occurred in South Africa and 180,000 patients died from AIDS-related illnesses in 2015 (UNAIDS gap report as cited in http://www.avert.org). In 2015 HIV prevalence in South Africa varied between regions from 18% to 40% amongst adults, despite the fact that the South African ART program is the largest one in the world (http://www.who.int/hiv/pub/arv/global-aids-update-2016-pub/en/). KS will therefore continue to be a major cancer burden during the next few years.
6. Diagnosis and Pathology
Although KS can be strongly suspected in an appropriate clinical setting, recent studies confirmed the limited predictive value of clinical diagnosis of KS [55]. Histopathological confirmation of a diagnosis of KS remains the gold standard, but the diagnosis is often not straight forward, especially if pathologists are not familiar with the spectrum of histopathological features of KS. Histopathological diagnosis of early stage KS depends on the detection of subtle clues that can be easily missed by the pathologist [56]. Well-established clinical lesions of KS however typically, but not always, display characteristic histopathological features that can be accurately diagnosed by a trained histopathologist [57–60].
The wide morphological spectrum of KS may mimic numerous unrelated non-neoplastic and neoplastic conditions, presenting a diagnostic pitfall to the pathologist. Pathologists should be aware of recognized variants of KS including anaplastic, telangiectatic, lymphangioma-like, cavernous hemangioma–like, pyogenic granuloma–like, intravascular, bullous, ecchymotic, hyperkeratotic, keloidal, micronodular, glomeruloid, solid, keloidal, desmoplastic, KS with myoid nodules, KS with sarcoidlike granulomas and pigmented KS [57–61]. Spindle-shaped cells are present in all forms of KS [62]. They represent a unifying feature, form the basis of diagnosis, and constitute the bulk of the proliferating cell fraction as ascertained by Ki-67 stain or by other molecular markers of proliferation.
In almost all KS biopsies spindle-shaped cells are infected by KSHV [63]. KSHV is necessary for KS development [26, 64]. All KSHV-infected cells transcribe so-called latent messenger RNAs and a minimal set of viral proteins [19, 21, 65]. The KSHV latency-associated nuclear antigen (LANA) has become the deciding diagnostic marker for KS. LANA-specific monoclonal antibodies are robust and are commercially available for automated immunohistochemical staining system. They are directed against a highly antigenic repeat motif in the center of the protein EQEQE. A positive LANA stain unequivocally confirms a diagnosis of KS in the appropriate clinicopathological context [66–69]. However, LANA expression may also be present in other KSHV associated conditions including multicentric Castleman’s disease, primary effusion lymphoma, and lymphoma arising in KSHV-associated multicentric Castleman’s disease [70]. LANA expression is therefore not confined to KS lesions [65, 71, 72]. KSHV DNAemia is commonly present in HIV+ patients with KS at the time of ART induction or with progressive KS, but undetectable in almost all patients on ART and those with regression of KS [19, 73].
LANA staining, however, is variable. It depends to some degree on the clinical progression stage of the disease, particularly in skin lesions. Superficial lesions or lesions that develop in patients on stable combination antiretroviral therapy (cART) may have very few LANA positive cells [74]. In patients with multiple lesions there can be a tendency to biopsy milder lesions to minimize bleeding and these tend to have fewer spindle cells and among those fewer LANA positive cells. The variable staining pattern for LANA in KS cells does not relate to patient age, gender, clinical subtype of KS, the distribution or extent of KS lesions, or CD4 count [75, 76]. Although stage of KS and the immunohistochemical method have been shown to influence variable staining for LANA in KS cells, the factors that influence the level of LANA expression remain unknown [76].
The absence of detectable LANA expression on immunohistochemistry may be due to technical reasons or very low viral copy numbers within KS cells. Failure to demonstrate LANA does not necessarily rule out KS in an appropriate clinicopathological setting [77]. In such cases, LANA-stained sections should be carefully re-assessed for subtle granular staining in KS cell nuclei [76]. PCR has been shown to reliably detect HHV-8 in KS lesions even in the absence of LANA expression [77, 78]. However, recent studies highlighted potential contamination of KS biopsies with subsequent false positive PCR results for HHV-8 [79]. Negative LANA expression should therefore always prompt very careful reconsideration of all clinicopathological features and potential alternative conditions should be considered in the differential diagnosis.
7. Update on treatment approaches
The FDA-approved treatment modalities for KS have not changed in 20 years. This may seem disappointing at first glance, but it also implies that we have 20 years of experience with the current standard of care. As KS manifests in many forms, therapies should also be divided into multiple application scenarios.
AIDS-KS responds to immune reconstitution and HIV suppression. Depending on geographic location and severity of presentation, 50% of AIDS-KS responds to cART [80–85]. State of the art cART and monitoring of its efficacy are essential in the treatment of AIDS-KS and often suffice. The goal is to reconstitute the immune system and suppress HIV replication, either in the context of treatment naive patients where KS lesions are the first indication of HIV infection, or in the context of AIDS, where KS lesions indicate HIV cART failure. A fraction of AIDS-KS responds to introduction of cART with disease progression. This phenomenon has been termed KS immune reconstitution syndrome (KS-IRIS) [86–88]. It is seen in as many as 10% of patients exposed to cART for the first time, particularly in Africa. The cause of this manifestation of KS is unclear. Clearly withdrawing cART is not an option, but concurrent chemotherapy and perhaps immune modulating adjuvants may be beneficial in the short-term [89]. Importantly though, both KS disease stabilization as well as disease acceleration have been reported in response to steroids [90, 91]. If and how other modulators of inflammation and immunoactivation would work is unknown.
There are several studies that suggest that HIV protease inhibitors, and in particular nelfinavir, have direct anti-KS activity in addition to their anti-retroviral therapy [88, 92–94]. There are also reports that fail to see a clinical benefit in KS comparing non-protease inhibitor containing regimen to protease inhibitor containing regimen [95]. Pre-clinical data on the efficacy of nelfinavir against KS and other solid tumors are encouraging, but it is difficult to judge how these would translate into clinical practice. Several trials of nelfinavir against KS and other solid cancers are ongoing, but no outcomes are available. In this regard it is noteworthy that many modern cART regimen no longer contain protease inhibitors. For instance, Gilead uses cobicistat in its NNRTI-based medication: elvitegravir/ cobicistat/ emtricitabine/ tenofovir disoproxil. Cobicistat like the protease inhibitor ritonavir boosts drug concentrations by inhibiting P450 metabolism, but has no protease inhibitory activity.
A third of AIDS-KS in the US now develops in the context of successful cART, i.e. in patients with undetectable HIV viral load and near-normal CD4 counts [96, 97]. We know that even these patients experience HIV disease, either in the form of unspecific, systemic immune activation or as a result of incomplete CD4 T cell receptor reconstitution after symptomatic HIV disease [98].
Transplant-KS responds to immune reconstitution, though lowering the immunosuppressive dose risks graft rejection. Here KSHV can be acquired before transplant, after transplant or through the transplant [4, 29, 99, 100]. Switching the chemical immune suppressive regimen from cyclosporine A/FK506 to mammalian target of rapamycin (mTOR) inhibitors such as rapamycin/sirolimus/everolimus often leads to KS regression [101, 102]. The mechanism of action is the subject of investigation. One difference between cyclosporine A and sirolimus is that cyclophillin, the target of cyclosporine is only expressed in T cells, whereas mTOR, the target of rapamycin is expressed in T cells, B cells, endothelial cells, and in most KS tumors. Even established KS lesions respond to rapamycin directly and independently of immune reconstitution in AIDS KS [74] and immunodeficient preclinical models [103], though rapamycin primarily stalls tumor growth leading to stable disease rather than inducing tumor regression outright.
Cytotoxic chemotherapy represents the standard of care for KS [104] including in children [105]. DNA damaging agents, such as doxorubicin are effective in 60–80% of KS in the US, which conversely implies that state of the art therapy fails in up to a third of KS patients (Figure 2). The median survival in response to cytotoxic chemotherapy is worse in resource-limited settings and populations with less than optimal access to HIV and cancer care (Figure 2). Liposomal formulations of doxorubicin or daunorubicine minimize systemic toxicity [106, 107], In addition, paclitaxel also shows clinical efficacy against KS [108–111]. It is often considered as second line therapy or used in situations where doxorubicin is not available.
VEGF is essential for endothelial proliferation and inhibition of VEGF constitutes a rational therapeutic approach for Kaposi Sarcoma. Thus far, however, clinical trials of single agent trials of VEGF neutralizing antibodies or VEGF-receptor inhibitors (bevacizumab, imatinib) have been ambiguous [112–115]. Anti-VEGF therapy worked in some patients, but not others. This variable results may be due to inadequacy of drug delivery to the KS lesion, the overall performance status of the patients, and redundancy in angiogenic factor signaling, as VEGF, bFGF and PDGF all contribute to KS growth [116, 117]. In other solid tumor indications angiogenesis inhibitors such as bevacizumab are used in the adjuvant setting and we speculate that this would have utility in KS as well. Further downstream acting drugs, such as the mTOR inhibitor sirolimus have shown clinical efficacy in transplant KS and it may be interesting to explore more potent VEGFR/c-kit inhibitors such as cabozantinib, pan-kinase inhibitors such as sorafenib, or anti-VEGF antibodies with different pharmacokinetics or intralesional applications.
IFN-alpha and its pegylated derivatives have been used with some success in the early days of the AIDS epidemic and against classic KS [118–121]. Doxil has now largely replaced IFN-alpha. It is noteworthy, however, in that the clinical success of pegylated IFN-alpha provided the first in human evidence for the extreme immune reactivity of KS. It has since been observed in the context of other immune modulatory drugs [122]. A fraction of KS, like a fraction of melanoma (reviewed in [123]), will spontaneously regress. Many, though not all, transplant KS as well as AIDS-KS lesions will regress upon immune reconstitution alone. The molecular mechanisms of these seemingly spontaneous regression events are unclear, but support further explorations in this area.
Imiquimod is a TLR7 agonist with non-specific activity against a variety of conditions. It is FDA approved as a topical formulation for the treatment of actinic keratosis and superficial basal cell carcinoma. Since 2015 generic forms of imiquimod are available. TLR7/8 agonists reactivate KSHV [124], but imiquimod was one of the weaker stimulants in this experimental system. If this reactivation leads to virus release, this in turn may stimulate tissue and tumor-resident antigen presenting cells [125]. At this point several cases of skin KS responding to topical treatment with imiquimod have been published, as have been treatment failures [126–128]. A systemic clinical trial has not been conducted.
Another group of agents, with promising clinical experiences [129–132] includes thalidomide and it’s more active derivatives pomalidomide and lenalidomide. These agents have broad immunomodulatory, anti-angiogenesis properties, targeting NFκB among others and are the subject of ongoing clinical studies. Like other immunomodulatory agents, one could speculate that their benefit will be most pronounced in the setting of limited KS, classic KS, or as adjuvant to chemotherapy.
Immune checkpoint inhibitors have gained notoriety because of their overwhelming efficacy in a select group of immunoreactive cancers, including melanoma and polyomavirus associated Merkel cell carcinoma [133–136]. It is likely that these agents will be also be active against KS, although clinical evidence has not been formally reported. Nivolumab is a humanized monoclonal antibody directed against PD-1, which is a negative co-receptor that is expressed on activated T cells. Ipilimumab is a humanized monoclonal antibody directed against CTL4. Small studies suggest that KS tumor cells do not express the T cell co-stimulatory molecules CD80 and CD86 [137]; the expression of the T cell inhibitory molecule PD-L1 and PD1 is variable and needs to be explored further [138, 139]. At present the AIDS malignancies clinical trials consortium (AMC) is conducting a phase I/II trial of nivolomab alone or in combination with ipilimumab in Kaposi Sarcoma (NCT02408861).
8. Summary and Outlook
In sum, Kaposi Sarcoma will continue to be seen at elevated frequencies in people living with HIV/ AIDS. It remains the most common cancer in regions that were endemic for KSHV and now experience substantial co-infection with HIV. People co-infected with HIV and KSHV are at risk of developing KS even if HIV is controlled by cART and this risk will increase as a person ages. The histopathological diagnosis of KS is not trivial, but greatly improved by the detection of LANA in biopsies of KS lesions. Detection of KSHV DNA or RNA is not yet developed to the same standard of accuracy. Doxil and Paclitaxel are efficacious against KS, but also associated with significant toxicity. Newer approaches such as sirolimus, VEGF/VEGF receptor inhibitors and immune modulatory agents show promise, but we do not yet know which types of KS and which kind of patients will benefit the most from these new agents. In the early days of the AIDS epidemic, skin KS was extensive and often accompanied by systemic KS, requiring systemic drug delivery. In the era of concurrent cART and complete HIV suppression, many HIV+ KS patients present with only localized lesions and may benefit from intralesional therapy alone or newer agents with more limited toxicity than standard dose chemotherapy.
Key points.
Kaposi Sarcoma continues to be among the most common causes of morbidity and mortality in people living with HIV worldwide.
There is evidence for multiple types of Kaposi Sarcoma, dependent on clinical presentation, cofactors and viral gene expression.
New approaches to Kaposi Sarcoma are under development.
Acknowledgments
We would like to thank our colleagues B. Damania, Marcia Sanders, Anthony Eason, for critical reading and insightful discussions.
Funding. This work is supported by PHS grants DE018304, CA190152 to DPD and CA192744 to JWS and DPD. The authors are members of the AIDS Malignancy Consortium (AMC), which is supported by PHS, grant CA121947 and JWS is a member of the AIDS Cancer Specimen Resource (ACSR), which is supported by PHS grant CA181255.
Footnotes
Compliance with Ethical Standards
Conflict of interest. DPD has received reagents from and was in a consulting agreement related to KS with Delenex AG and Navidea Inc. This did not influence the opinion expressed in this review.
References
- 1.Curtiss P, Strazzulla LC, Friedman-Kien AE. An Update on Kaposi’s Sarcoma: Epidemiology, Pathogenesis and Treatment. Dermatol Ther (Heidelb) 2016 Nov 1; doi: 10.1007/s13555-016-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Mallawany NK, Kamiyango W, Slone JS, Villiera J, Kovarik CL, Cox CM, et al. Clinical Factors Associated with Long-Term Complete Remission versus Poor Response to Chemotherapy in HIV-Infected Children and Adolescents with Kaposi Sarcoma Receiving Bleomycin and Vincristine: A Retrospective Observational Study. PLoS One. 2016;11(4):e0153335. doi: 10.1371/journal.pone.0153335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefan DC. Patterns of distribution of childhood cancer in Africa. J Trop Pediatr. 2015 Jun;61(3):165–73. doi: 10.1093/tropej/fmv005. [DOI] [PubMed] [Google Scholar]
- 4.Parravicini C, Olsen SJ, Capra M, Poli F, Sirchia G, Gao SJ, et al. Risk of Kaposi’s sarcoma-associated herpes virus transmission from donor allografts among Italian posttransplant Kaposi’s sarcoma patients. Blood. 1997 Oct 1;90(7):2826–9. [PubMed] [Google Scholar]
- 5.Bower M, Nelson M, Young AM, Thirlwell C, Newsom-Davis T, Mandalia S, et al. Immune reconstitution inflammatory syndrome associated with Kaposi’s sarcoma. J Clin Oncol. 2005 Aug 1;23(22):5224–8. doi: 10.1200/JCO.2005.14.597. [DOI] [PubMed] [Google Scholar]
- 6.Jackson CC, Dickson MA, Sadjadi M, Gessain A, Abel L, Jouanguy E, et al. Kaposi Sarcoma of Childhood: Inborn or Acquired Immunodeficiency to Oncogenic HHV-8. Pediatr Blood Cancer. 2016 Mar;63(3):392–7. doi: 10.1002/pbc.25779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Northfelt DW, Dezube BJ, Thommes JA, Levine R, Von Roenn JH, Dosik GM, et al. Efficacy of pegylated-liposomal doxorubicin in the treatment of AIDS-related Kaposi’s sarcoma after failure of standard chemotherapy. J Clin Oncol. 1997 Feb;15(2):653–9. doi: 10.1200/JCO.1997.15.2.653. [DOI] [PubMed] [Google Scholar]
- 8.Cianfrocca M, Lee S, Von Roenn J, Tulpule A, Dezube BJ, Aboulafia DM, et al. Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: evidence of symptom palliation from chemotherapy. Cancer. 2010 Aug 15;116(16):3969–77. doi: 10.1002/cncr.25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelbrecht S, Treurnicht FK, Schneider JW, Jordaan HF, Steytler JG, Wranz PA, et al. Detection of human herpes virus 8 DNA and sequence polymorphism in classical, epidemic, and iatrogenic Kaposi’s sarcoma in South Africa. J Med Virol. 1997 Jun;52(2):168–72. [PubMed] [Google Scholar]
- 10.Moore PS, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and without HIV infection. N Engl J Med. 1995 May 4;332(18):1181–5. doi: 10.1056/NEJM199505043321801. [DOI] [PubMed] [Google Scholar]
- 11.Huang YQ, Li JJ, Kaplan MH, Poiesz B, Katabira E, Zhang WC, et al. Human herpesvirus-like nucleic acid in various forms of Kaposi’s sarcoma. Lancet. 1995 Mar 25;345(8952):759–61. doi: 10.1016/s0140-6736(95)90641-x. [DOI] [PubMed] [Google Scholar]
- 12.Dupin N, Grandadam M, Calvez V, Gorin I, Aubin JT, Havard S, et al. Herpesvirus-like DNA sequences in patients with Mediterranean Kaposi’s sarcoma. Lancet. 1995 Mar 25;345(8952):761–2. doi: 10.1016/s0140-6736(95)90642-8. [DOI] [PubMed] [Google Scholar]
- 13.Chuck S, Grant RM, Katongole-Mbidde E, Conant M, Ganem D. Frequent presence of a novel herpesvirus genome in lesions of human immunodeficiency virus-negative Kaposi’s sarcoma. J Infect Dis. 1996 Jan;173(1):248–51. doi: 10.1093/infdis/173.1.248. [DOI] [PubMed] [Google Scholar]
- 14.Zong J, Ciufo DM, Viscidi R, Alagiozoglou L, Tyring S, Rady P, et al. Genotypic analysis at multiple loci across Kaposi’s sarcoma herpesvirus (KSHV) DNA molecules: clustering patterns, novel variants and chimerism. J Clin Virol. 2002 Jan;23(3):119–48. doi: 10.1016/s1386-6532(01)00205-0. [DOI] [PubMed] [Google Scholar]
- 15.Treurnicht FK, Engelbrecht S, Taylor MB, Schneider JW, van Rensburg EJ. HHV-8 subtypes in South Africa: identification of a case suggesting a novel B variant. J Med Virol. 2002 Feb;66(2):235–40. doi: 10.1002/jmv.2135. [DOI] [PubMed] [Google Scholar]
- 16.Isaacs T, Abera AB, Muloiwa R, Katz AA, Todd G. Genetic diversity of HHV8 subtypes in South Africa: A5 subtype is associated with extensive disease in AIDS-KS. J Med Virol. 2016 Feb;88(2):292–303. doi: 10.1002/jmv.24328. [DOI] [PubMed] [Google Scholar]
- 17.Dittmer DP, Damania B. Kaposi sarcoma-associated herpesvirus: immunobiology, oncogenesis, and therapy. J Clin Invest. 2016 Sep 1;126(9):3165–75. doi: 10.1172/JCI84418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung J, Munz C. Immune control of oncogenic gamma-herpesviruses. Curr Opin Virol. 2015 Oct;14:79–86. doi: 10.1016/j.coviro.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosseinipour MC, Sweet KM, Xiong J, Namarika D, Mwafongo A, Nyirenda M, et al. Viral profiling identifies multiple subtypes of Kaposi’s sarcoma. MBio. 2014 Sep 23;5(5):e01633–14. doi: 10.1128/mBio.01633-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Hara AJ, Chugh P, Wang L, Netto EM, Luz E, Harrington WJ, et al. Pre-micro RNA signatures delineate stages of endothelial cell transformation in Kaposi sarcoma. PLoS Pathog. 2009 Apr;5(4):e1000389. doi: 10.1371/journal.ppat.1000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dittmer DP. Transcription profile of Kaposi’s sarcoma-associated herpesvirus in primary Kaposi’s sarcoma lesions as determined by real-time PCR arrays. Cancer Res. 2003 May 1;63(9):2010–5. [PubMed] [Google Scholar]
- 22.Ballestas ME, Chatis PA, Kaye KM. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science. 1999 Apr 23;284(5414):641–4. doi: 10.1126/science.284.5414.641. [DOI] [PubMed] [Google Scholar]
- 23.Martin DF, Kuppermann BD, Wolitz RA, Palestine AG, Li H, Robinson CA. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N Engl J Med. 1999 Apr 8;340(14):1063–70. doi: 10.1056/NEJM199904083401402. [DOI] [PubMed] [Google Scholar]
- 24.Casper C, Krantz E, Selke S, Kuntz SR, Wang J, Huang ML, et al. Frequent and asymptomatic oropharyngeal shedding of human herpesvirus 8 among immunocompetent men. J Infect Dis. 2007 Jan 1;195(1):30–6. doi: 10.1086/509621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bender Ignacio RA, Goldman JD, Magaret AS, Selke S, Huang ML, Gantt S, et al. Patterns of human herpesvirus-8 oral shedding among diverse cohorts of human herpesvirus-8 seropositive persons. Infect Agent Cancer. 2016;11:7. doi: 10.1186/s13027-016-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin JN, Ganem DE, Osmond DH, Page-Shafer KA, Macrae D, Kedes DH. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998 Apr 2;338(14):948–54. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 27.Butler LM, Osmond DH, Jones AG, Martin JN. Use of saliva as a lubricant in anal sexual practices among homosexual men. J Acquir Immune Defic Syndr. 2009 Feb 1;50(2):162–7. doi: 10.1097/QAI.0b013e31819388a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kedes DH, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. The seroepidemiology of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat Med. 1996 Aug;2(8):918–24. doi: 10.1038/nm0896-918. [DOI] [PubMed] [Google Scholar]
- 29.Barozzi P, Luppi M, Facchetti F, Mecucci C, Alu M, Sarid R, et al. Post-transplant Kaposi sarcoma originates from the seeding of donor-derived progenitors. Nat Med. 2003 May;9(5):554–61. doi: 10.1038/nm862. [DOI] [PubMed] [Google Scholar]
- 30.Group ISS, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015 Aug 27;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ensoli B, Sturzl M, Monini P. Reactivation and role of HHV-8 in Kaposi’s sarcoma initiation. Adv Cancer Res. 2001;81:161–200. doi: 10.1016/s0065-230x(01)81005-8. [DOI] [PubMed] [Google Scholar]
- 32.Staudt MR, Kanan Y, Jeong JH, Papin JF, Hines-Boykin R, Dittmer DP. The tumor microenvironment controls primary effusion lymphoma growth in vivo. Cancer Res. 2004 Jul 15;64(14):4790–9. doi: 10.1158/0008-5472.CAN-03-3835. [DOI] [PubMed] [Google Scholar]
- 33.Krown SE, Dittmer DP, Cesarman E. Pilot study of oral valganciclovir therapy in patients with classic Kaposi sarcoma. J Infect Dis. 2011 Apr 15;203(8):1082–6. doi: 10.1093/infdis/jiq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unemori P, Leslie KS, Hunt PW, Sinclair E, Epling L, Mitsuyasu R, et al. Immunosenescence is associated with presence of Kaposi’s sarcoma in antiretroviral treated HIV infection. AIDS. 2013 Jul 17;27(11):1735–42. doi: 10.1097/QAD.0b013e3283601144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong YK, Foreman K, Shin JW, Hirakawa S, Curry CL, Sage DR, et al. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat Genet. 2004 Jul;36(7):683–5. doi: 10.1038/ng1383. [DOI] [PubMed] [Google Scholar]
- 36.Wang HW, Trotter MW, Lagos D, Bourboulia D, Henderson S, Makinen T, et al. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat Genet. 2004 Jul;36(7):687–93. doi: 10.1038/ng1384. [DOI] [PubMed] [Google Scholar]
- 37.Ojala PM, Schulz TF. Manipulation of endothelial cells by KSHV: implications for angiogenesis and aberrant vascular differentiation. Semin Cancer Biol. 2014 Jun;26:69–77. doi: 10.1016/j.semcancer.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Masood R, Xia G, Smith DL, Scalia P, Still JG, Tulpule A, et al. Ephrin B2 expression in Kaposi sarcoma is induced by human herpesvirus type 8: phenotype switch from venous to arterial endothelium. Blood. 2005 Feb 1;105(3):1310–8. doi: 10.1182/blood-2004-03-0933. [DOI] [PubMed] [Google Scholar]
- 39.Liu R, Li X, Tulpule A, Zhou Y, Scehnet JS, Zhang S, et al. KSHV-induced notch components render endothelial and mural cell characteristics and cell survival. Blood. 2010 Jan 28;115(4):887–95. doi: 10.1182/blood-2009-08-236745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGrath MS, Shiramizu BT, Herndier BG. Identification of a clonal form of HIV in early Kaposi’s sarcoma: evidence for a novel model of oncogenesis, “sequential neoplasia”. J Acquir Immune Defic Syndr Hum Retrovirol. 1995 Apr 1;8(4):379–85. [PubMed] [Google Scholar]
- 41.Cheng F, Pekkonen P, Laurinavicius S, Sugiyama N, Henderson S, Gunther T, et al. KSHV-initiated notch activation leads to membrane-type-1 matrix metalloproteinase-dependent lymphatic endothelial-to-mesenchymal transition. Cell Host Microbe. 2011 Dec 15;10(6):577–90. doi: 10.1016/j.chom.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Matthews DD, Herrick AL, Coulter RW, Friedman MR, Mills TC, Eaton LA, et al. Running Backwards: Consequences of Current HIV Incidence Rates for the Next Generation of Black MSM in the United States. AIDS Behav. 2016 Jan;20(1):7–16. doi: 10.1007/s10461-015-1158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parkin DM, Bray F, Ferlay J, Jemal A. Cancer in Africa 2012. Cancer Epidemiol Biomarkers Prev. 2014 Jun;23(6):953–66. doi: 10.1158/1055-9965.EPI-14-0281. [DOI] [PubMed] [Google Scholar]
- 44.Jemal A, Bray F, Forman D, O’Brien M, Ferlay J, Center M, et al. Cancer burden in Africa and opportunities for prevention. Cancer. 2012 Sep 15;118(18):4372–84. doi: 10.1002/cncr.27410. [DOI] [PubMed] [Google Scholar]
- 45.Rogena EA, Simbiri KO, De Falco G, Leoncini L, Ayers L, Nyagol J. A review of the pattern of AIDS defining, HIV associated neoplasms and premalignant lesions diagnosed from 2000–2011 at Kenyatta National Hospital, Kenya. Infect Agent Cancer. 2015;10:28. doi: 10.1186/s13027-015-0021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semeere A, Wenger M, Busakhala N, Buziba N, Bwana M, Muyindike W, et al. A prospective ascertainment of cancer incidence in sub-Saharan Africa: The case of Kaposi sarcoma. Cancer Med. 2016 May;5(5):914–28. doi: 10.1002/cam4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dollard SC, Butler LM, Jones AM, Mermin JH, Chidzonga M, Chipato T, et al. Substantial regional differences in human herpesvirus 8 seroprevalence in sub-Saharan Africa: insights on the origin of the “Kaposi’s sarcoma belt”. Int J Cancer. 2010 Nov 15;127(10):2395–401. doi: 10.1002/ijc.25235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wabinga HR, Nambooze S, Amulen PM, Okello C, Mbus L, Parkin DM. Trends in the incidence of cancer in Kampala, Uganda 1991–2010. Int J Cancer. 2014 Jul 15;135(2):432–9. doi: 10.1002/ijc.28661. [DOI] [PubMed] [Google Scholar]
- 49.Chokunonga E, Borok MZ, Chirenje ZM, Nyakabau AM, Parkin DM. Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991–2010. Int J Cancer. 2013 Aug 1;133(3):721–9. doi: 10.1002/ijc.28063. [DOI] [PubMed] [Google Scholar]
- 50.Chokunonga E, Windridge P, Sasieni P, Borok M, Parkin DM. Black-white differences in cancer risk in Harare, Zimbabwe, during 1991–2010. Int J Cancer. 2016 Mar 15;138(6):1416–21. doi: 10.1002/ijc.29883. [DOI] [PubMed] [Google Scholar]
- 51.Carrilho C, Ferro J, Lorenzoni C, Sultane T, Silva-Matos C, Lunet N. A contribution for a more accurate estimation of the incidence of Kaposi sarcoma in Mozambique. Int J Cancer. 2013 Feb 15;132(4):988–9. doi: 10.1002/ijc.27714. [DOI] [PubMed] [Google Scholar]
- 52.Robey RC, Bower M. Facing up to the ongoing challenge of Kaposi’s sarcoma. Curr Opin Infect Dis. 2015 Feb;28(1):31–40. doi: 10.1097/QCO.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 53.Bohlius J, Valeri F, Maskew M, Prozesky H, Garone D, Sengayi M, et al. Kaposi’s Sarcoma in HIV-infected patients in South Africa: Multicohort study in the antiretroviral therapy era. Int J Cancer. 2014 Dec 1;135(11):2644–52. doi: 10.1002/ijc.28894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davidson A, Wainwright RD, Stones DK, Kruger M, Hendricks M, Geel J, et al. Malignancies in South African children with HIV. J Pediatr Hematol Oncol. 2014 Mar;36(2):111–7. doi: 10.1097/MPH.0b013e31829cdd49. [DOI] [PubMed] [Google Scholar]
- 55.Amerson E, Woodruff CM, Forrestel A, Wenger M, McCalmont T, LeBoit P, et al. Accuracy of Clinical Suspicion and Pathologic Diagnosis of Kaposi Sarcoma in East Africa. J Acquir Immune Defic Syndr. 2016 Mar 1;71(3):295–301. doi: 10.1097/QAI.0000000000000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ackerman AB. Subtle clues to diagnosis by conventional microscopy. The patch stage of Kaposi’s sarcoma. Am J Dermatopathol. 1979 Summer;1(2):165–72. doi: 10.1097/00000372-197900120-00011. [DOI] [PubMed] [Google Scholar]
- 57.Luzar B, Antony F, Ramdial PK, Calonje E. Intravascular Kaposi’s sarcoma - a hitherto unrecognized phenomenon. J Cutan Pathol. 2007 Nov;34(11):861–4. doi: 10.1111/j.1600-0560.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- 58.Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008 Jul 25;3:31. doi: 10.1186/1746-1596-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Donnell PJ, Pantanowitz L, Grayson W. Unique histologic variants of cutaneous Kaposi sarcoma. Am J Dermatopathol. 2010 May;32(3):244–50. doi: 10.1097/DAD.0b013e3181b7f6a7. [DOI] [PubMed] [Google Scholar]
- 60.Bunn BK, de Carvalho MV, Louw M, Vargas PA, van Heerden WF. Microscopic diversity in oral Kaposi sarcoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013 Feb;115(2):241–8. doi: 10.1016/j.oooo.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 61.Sutton AM, Tarbox M, Burkemper NM. Cavernous hemangioma-like Kaposi sarcoma: a unique histopathologic variant. Am J Dermatopathol. 2014 May;36(5):440–2. doi: 10.1097/DAD.0b013e3182974502. [DOI] [PubMed] [Google Scholar]
- 62.Sturzl M, Brandstetter H, Roth WK. Kaposi’s sarcoma: a review of gene expression and ultrastructure of KS spindle cells in vivo. AIDS Res Hum Retroviruses. 1992 Oct;8(10):1753–63. doi: 10.1089/aid.1992.8.1753. [DOI] [PubMed] [Google Scholar]
- 63.Staskus KA, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, et al. Kaposi’s sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997 Jan;71(1):715–9. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao SJ, Kingsley L, Hoover DR, Spira TJ, Rinaldo CR, Saah A, et al. Seroconversion to antibodies against Kaposi’s sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi’s sarcoma. N Engl J Med. 1996 Jul 25;335(4):233–41. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- 65.Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, et al. Distribution of human herpesvirus-8 latently infected cells in Kaposi’s sarcoma, multicentric Castleman’s disease, and primary effusion lymphoma. Proc Natl Acad Sci U S A. 1999 Apr 13;96(8):4546–51. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robin YM, Guillou L, Michels JJ, Coindre JM. Human herpesvirus 8 immunostaining: a sensitive and specific method for diagnosing Kaposi sarcoma in paraffin-embedded sections. Am J Clin Pathol. 2004 Mar;121(3):330–4. doi: 10.1309/96U1-6LRR-AN5H-WWVE. [DOI] [PubMed] [Google Scholar]
- 67.Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004 Apr;17(4):456–60. doi: 10.1038/modpathol.3800061. [DOI] [PubMed] [Google Scholar]
- 68.Hong A, Davies S, Stevens G, Lee CS. Cyclin D1 overexpression in AIDS-related and classic Kaposi sarcoma. Appl Immunohistochem Mol Morphol. 2004 Mar;12(1):26–30. doi: 10.1097/00129039-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Pereira PF, Cuzzi T, Galhardo MC. Immunohistochemical detection of the latent nuclear antigen-1 of the human herpesvirus type 8 to differentiate cutaneous epidemic Kaposi sarcoma and its histological simulators. An Bras Dermatol. 2013 Mar-Apr;88(2):243–6. doi: 10.1590/S0365-05962013000200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cesarman E. Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Lett. 2011 Jun 28;305(2):163–74. doi: 10.1016/j.canlet.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pantanowitz L, Dezube BJ, Pinkus GS, Tahan SR. Histological characterization of regression in acquired immunodeficiency syndrome-related Kaposi’s sarcoma. J Cutan Pathol. 2004 Jan;31(1):26–34. doi: 10.1046/j.0303-6987.2004.0132.x. [DOI] [PubMed] [Google Scholar]
- 72.Pantanowitz L, Pinkus GS, Dezube BJ, Tahan SR. HHV8 is not limited to Kaposi’s sarcoma. Mod Pathol. 2005 Aug;18(8):1148. doi: 10.1038/modpathol.3800441. author reply 9–50. [DOI] [PubMed] [Google Scholar]
- 73.Broccolo F, Tassan Din C, Vigano MG, Rutigliano T, Esposito S, Lusso P, et al. HHV-8 DNA replication correlates with the clinical status in AIDS-related Kaposi’s sarcoma. J Clin Virol. 2016 May;78:47–52. doi: 10.1016/j.jcv.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 74.Krown SE, Roy D, Lee JY, Dezube BJ, Reid EG, Venkataramanan R, et al. Rapamycin with antiretroviral therapy in AIDS-associated Kaposi sarcoma: an AIDS Malignancy Consortium study. J Acquir Immune Defic Syndr. 2012 Apr 15;59(5):447–54. doi: 10.1097/QAI.0b013e31823e7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hong A, Davies S, Lee CS. Immunohistochemical detection of the human herpes virus 8 (HHV8) latent nuclear antigen-1 in Kaposi’s sarcoma. Pathology. 2003 Oct;35(5):448–50. doi: 10.1080/00313020310001602657. [DOI] [PubMed] [Google Scholar]
- 76.Mohanlal RD, Pather S. Variability of HHV8 LNA-1 Immunohistochemical Staining Across the 3 Histologic Stages of HIV-Associated Mucocutaneous Kaposi Sarcoma: Is There a Relationship to Patients’ CD4 Counts? Am J Dermatopathol. 2015 Jul;37(7):530–4. doi: 10.1097/DAD.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 77.Pak F, Pyakural P, Kokhaei P, Kaaya E, Pourfathollah AA, Selivanova G, et al. HHV-8/KSHV during the development of Kaposi’s sarcoma: evaluation by polymerase chain reaction and immunohistochemistry. J Cutan Pathol. 2005 Jan;32(1):21–7. doi: 10.1111/j.0303-6987.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 78.Snodgrass R, Gardner A, Jiang L, Fu C, Cesarman E, Erickson D. KS-Detect - Validation of Solar Thermal PCR for the Diagnosis of Kaposi’s Sarcoma Using Pseudo-Biopsy Samples. PLoS One. 2016;11(1):e0147636. doi: 10.1371/journal.pone.0147636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Speicher DJ, Wanzala P, D’Lima M, Njiru A, Chindia M, Dimba E, et al. Diagnostic challenges of oral and cutaneous Kaposi’s sarcoma in resource-constrained settings. J Oral Pathol Med. 2015 Nov;44(10):842–9. doi: 10.1111/jop.12315. [DOI] [PubMed] [Google Scholar]
- 80.Krown SE, Borok MZ, Campbell TB, Casper C, Dittmer DP, Hosseinipour MC, et al. Stage-stratified approach to AIDS-related Kaposi’s sarcoma: implications for resource-limited environments. J Clin Oncol. 2014 Aug 10;32(23):2512–3. doi: 10.1200/JCO.2014.55.8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stebbing J, Sanitt A, Nelson M, Powles T, Gazzard B, Bower M. A prognostic index for AIDS-associated Kaposi’s sarcoma in the era of highly active antiretroviral therapy. Lancet. 2006 May 6;367(9521):1495–502. doi: 10.1016/S0140-6736(06)68649-2. [DOI] [PubMed] [Google Scholar]
- 82.Krell J, Stebbing J. Broader implications of a stage-guided stratified therapeutic approach for AIDS-related Kaposi’s sarcoma. J Clin Oncol. 2014 Feb 10;32(5):373–5. doi: 10.1200/JCO.2013.53.7126. [DOI] [PubMed] [Google Scholar]
- 83.Bihl F, Mosam A, Henry LN, Chisholm JV, 3rd, Dollard S, Gumbi P, et al. Kaposi’s sarcoma-associated herpesvirus-specific immune reconstitution and antiviral effect of combined HAART/chemotherapy in HIV clade C-infected individuals with Kaposi’s sarcoma. AIDS. 2007 Jun 19;21(10):1245–52. doi: 10.1097/QAD.0b013e328182df03. [DOI] [PubMed] [Google Scholar]
- 84.Chinula L, Moses A, Gopal S. HIV-associated malignancies in sub-Saharan Africa: progress, challenges, and opportunities. Curr Opin HIV AIDS. 2016 Sep 7; doi: 10.1097/COH.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Semeere AS, Busakhala N, Martin JN. Impact of antiretroviral therapy on the incidence of Kaposi’s sarcoma in resource-rich and resource-limited settings. Curr Opin Oncol. 2012 Sep;24(5):522–30. doi: 10.1097/CCO.0b013e328355e14b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Letang E, Lewis JJ, Bower M, Mosam A, Borok M, Campbell TB, et al. Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma: higher incidence and mortality in Africa than in the UK. AIDS. 2013 Jun 19;27(10):1603–13. doi: 10.1097/QAD.0b013e328360a5a1. [DOI] [PubMed] [Google Scholar]
- 87.Achenbach CJ, Harrington RD, Dhanireddy S, Crane HM, Casper C, Kitahata MM. Paradoxical immune reconstitution inflammatory syndrome in HIV-infected patients treated with combination antiretroviral therapy after AIDS-defining opportunistic infection. Clin Infect Dis. 2012 Feb 1;54(3):424–33. doi: 10.1093/cid/cir802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kowalkowski MA, Kramer JR, Richardson PR, Suteria I, Chiao EY. Use of boosted protease inhibitors reduces Kaposi sarcoma incidence among male veterans with HIV infection. Clin Infect Dis. 2015 May 1;60(9):1405–14. doi: 10.1093/cid/civ012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Speicher DJ, Sehu MM, Johnson NW, Shaw DR. Successful treatment of an HIV-positive patient with unmasking Kaposi’s sarcoma immune reconstitution inflammatory syndrome. J Clin Virol. 2013 Jul;57(3):282–5. doi: 10.1016/j.jcv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 90.Catricala C, Marenda S, Muscardin LM, Donati P, Lepri A, Eibenschutz L. Angiomatous reaction Kaposi-sarcoma-like as a side effect of topical corticosteroid therapy in lichen sclerosus of the penis. Dermatol Ther. 2009 Jul-Aug;22(4):379–82. doi: 10.1111/j.1529-8019.2009.01249.x. [DOI] [PubMed] [Google Scholar]
- 91.Trattner A, Hodak E, David M, Sandbank M. The appearance of Kaposi sarcoma during corticosteroid therapy. Cancer. 1993 Sep 1;72(5):1779–83. doi: 10.1002/1097-0142(19930901)72:5<1779::aid-cncr2820720543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 92.Gantt S, Cattamanchi A, Krantz E, Magaret A, Selke S, Kuntz SR, et al. Reduced human herpesvirus-8 oropharyngeal shedding associated with protease inhibitor-based antiretroviral therapy. J Clin Virol. 2014 Jun;60(2):127–32. doi: 10.1016/j.jcv.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gantt S, Carlsson J, Ikoma M, Gachelet E, Gray M, Geballe AP, et al. The HIV protease inhibitor nelfinavir inhibits Kaposi’s sarcoma-associated herpesvirus replication in vitro. Antimicrob Agents Chemother. 2011 Jun;55(6):2696–703. doi: 10.1128/AAC.01295-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sgadari C, Monini P, Barillari G, Ensoli B. Use of HIV protease inhibitors to block Kaposi’s sarcoma and tumour growth. Lancet Oncol. 2003 Sep;4(9):537–47. doi: 10.1016/s1470-2045(03)01192-6. [DOI] [PubMed] [Google Scholar]
- 95.Bruyand M, Ryom L, Shepherd L, Fatkenheuer G, Grulich A, Reiss P, et al. Cancer risk and use of protease inhibitor or nonnucleoside reverse transcriptase inhibitor-based combination antiretroviral therapy: the D: A: D study. J Acquir Immune Defic Syndr. 2015 Apr 15;68(5):568–77. doi: 10.1097/QAI.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 96.Krown SE, Lee JY, Dittmer DP Consortium AM. More on HIV-associated Kaposi’s sarcoma. N Engl J Med. 2008 Jan 31;358(5):535–6. doi: 10.1056/NEJMc072994. author reply 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maurer T, Ponte M, Leslie K. HIV-associated Kaposi’s sarcoma with a high CD4 count and a low viral load. N Engl J Med. 2007 Sep 27;357(13):1352–3. doi: 10.1056/NEJMc070508. [DOI] [PubMed] [Google Scholar]
- 98.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009 Apr 15;199(8):1177–85. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luppi M, Barozzi P, Santagostino G, Trovato R, Schulz TF, Marasca R, et al. Molecular evidence of organ-related transmission of Kaposi sarcoma-associated herpesvirus or human herpesvirus-8 in transplant patients. Blood. 2000 Nov 1;96(9):3279–81. [PubMed] [Google Scholar]
- 100.Moosa MR, Treurnicht FK, van Rensburg EJ, Schneider JW, Jordaan HF, Engelbrecht S. Detection and subtyping of human herpesvirus-8 in renal transplant patients before and after remission of Kaposi’s sarcoma. Transplantation. 1998 Jul 27;66(2):214–8. doi: 10.1097/00007890-199807270-00013. [DOI] [PubMed] [Google Scholar]
- 101.Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, et al. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J Med. 2005 Mar 31;352(13):1317–23. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- 102.Nichols LA, Adang LA, Kedes DH. Rapamycin blocks production of KSHV/HHV8: insights into the anti-tumor activity of an immunosuppressant drug. PLoS One. 2011 Jan 14;6(1):e14535. doi: 10.1371/journal.pone.0014535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roy D, Sin SH, Lucas A, Venkataramanan R, Wang L, Eason A, et al. mTOR inhibitors block Kaposi sarcoma growth by inhibiting essential autocrine growth factors and tumor angiogenesis. Cancer Res. 2013 Apr 1;73(7):2235–46. doi: 10.1158/0008-5472.CAN-12-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chagaluka G, Stanley C, Banda K, Depani S, Nijram’madzi J, Katangwe T, et al. Kaposi’s sarcoma in children: an open randomised trial of vincristine, oral etoposide and a combination of vincristine and bleomycin. Eur J Cancer. 2014 May;50(8):1472–81. doi: 10.1016/j.ejca.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 105.Molyneux E, Davidson A, Orem J, Hesseling P, Balagadde-Kambugu J, Githanga J, et al. The management of children with Kaposi sarcoma in resource limited settings. Pediatr Blood Cancer. 2013 Apr;60(4):538–42. doi: 10.1002/pbc.24408. [DOI] [PubMed] [Google Scholar]
- 106.Mohrbacher AF, Gregory SA, Gabriel DA, Rusk JM, Giles FJ. Liposomal daunorubicin (DaunoXome) plus dexamethasone for patients with multiple myeloma. A phase II International Oncology Study Group study. Cancer. 2002 May 15;94(10):2645–52. doi: 10.1002/cncr.10561. [DOI] [PubMed] [Google Scholar]
- 107.Rosenthal E, Poizot-Martin I, Saint-Marc T, Spano JP, Cacoub P, Group DNXS. Phase IV study of liposomal daunorubicin (DaunoXome) in AIDS-related Kaposi sarcoma. Am J Clin Oncol. 2002 Feb;25(1):57–9. doi: 10.1097/00000421-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 108.Stebbing J, Wildfire A, Portsmouth S, Powles T, Thirlwell C, Hewitt P, et al. Paclitaxel for anthracycline-resistant AIDS-related Kaposi’s sarcoma: clinical and angiogenic correlations. Ann Oncol. 2003 Nov;14(11):1660–6. doi: 10.1093/annonc/mdg461. [DOI] [PubMed] [Google Scholar]
- 109.Gbabe OF, Okwundu CI, Dedicoat M, Freeman EE. Treatment of severe or progressive Kaposi’s sarcoma in HIV-infected adults. Cochrane Database Syst Rev. 2014;(9):CD003256. doi: 10.1002/14651858.CD003256.pub2. [DOI] [PubMed] [Google Scholar]
- 110.Raimundo K, Biskupiak J, Goodman M, Silverstein S, Asche C. Cost effectiveness of liposomal doxorubicin vs. paclitaxel for the treatment of advanced AIDS-Kaposi’s sarcoma. J Med Econ. 2013;16(5):606–13. doi: 10.3111/13696998.2013.777347. [DOI] [PubMed] [Google Scholar]
- 111.Gill PS, Tulpule A, Espina BM, Cabriales S, Bresnahan J, Ilaw M, et al. Paclitaxel is safe and effective in the treatment of advanced AIDS-related Kaposi’s sarcoma. J Clin Oncol. 1999 Jun;17(6):1876–83. doi: 10.1200/JCO.1999.17.6.1876. [DOI] [PubMed] [Google Scholar]
- 112.Uldrick TS, Wyvill KM, Kumar P, O’Mahony D, Bernstein W, Aleman K, et al. Phase II study of bevacizumab in patients with HIV-associated Kaposi’s sarcoma receiving antiretroviral therapy. J Clin Oncol. 2012 May 1;30(13):1476–83. doi: 10.1200/JCO.2011.39.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Koon HB, Bubley GJ, Pantanowitz L, Masiello D, Smith B, Crosby K, et al. Imatinib-induced regression of AIDS-related Kaposi’s sarcoma. J Clin Oncol. 2005 Feb 10;23(5):982–9. doi: 10.1200/JCO.2005.06.079. [DOI] [PubMed] [Google Scholar]
- 114.Koon HB, Krown SE, Lee JY, Honda K, Rapisuwon S, Wang Z, et al. Phase II trial of imatinib in AIDS-associated Kaposi’s sarcoma: AIDS Malignancy Consortium Protocol 042. J Clin Oncol. 2014 Feb 10;32(5):402–8. doi: 10.1200/JCO.2012.48.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bender Ignacio RA, Lee JY, Rudek MA, Dittmer DP, Ambinder RF, Krown SE, et al. Brief Report: A Phase 1b/Pharmacokinetic Trial of PTC299, a Novel PostTranscriptional VEGF Inhibitor, for AIDS-Related Kaposi’s Sarcoma: AIDS Malignancy Consortium Trial 059. J Acquir Immune Defic Syndr. 2016 May 1;72(1):52–7. doi: 10.1097/QAI.0000000000000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cavallin LE, Goldschmidt-Clermont P, Mesri EA. Molecular and cellular mechanisms of KSHV oncogenesis of Kaposi’s sarcoma associated with HIV/AIDS. PLoS Pathog. 2014 Jul;10(7):e1004154. doi: 10.1371/journal.ppat.1004154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Samaniego F, Markham PD, Gendelman R, Watanabe Y, Kao V, Kowalski K, et al. Vascular endothelial growth factor and basic fibroblast growth factor present in Kaposi’s sarcoma (KS) are induced by inflammatory cytokines and synergize to promote vascular permeability and KS lesion development. Am J Pathol. 1998 Jun;152(6):1433–43. [PMC free article] [PubMed] [Google Scholar]
- 118.Rybojad M, Borradori L, Verola O, Zeller J, Puissant A, Morel P. Non-AIDS-associated Kaposi’s sarcoma (classical and endemic African types): treatment with low doses of recombinant interferon-alpha. J Invest Dermatol. 1990 Dec;95(6 Suppl):176S–9S. doi: 10.1016/s0022-202x(90)91216-x. [DOI] [PubMed] [Google Scholar]
- 119.Krown SE, Paredes J, Bundow D, Polsky B, Gold JW, Flomenberg N. Interferon-alpha, zidovudine, and granulocyte-macrophage colony-stimulating factor: a phase I AIDS Clinical Trials Group study in patients with Kaposi’s sarcoma associated with AIDS. J Clin Oncol. 1992 Aug;10(8):1344–51. doi: 10.1200/JCO.1992.10.8.1344. [DOI] [PubMed] [Google Scholar]
- 120.Deichmann M, Thome M, Jackel A, Utermann S, Bock M, Waldmann V, et al. Non-human immunodeficiency virus Kaposi’s sarcoma can be effectively treated with low-dose interferon-alpha despite the persistence of herpesvirus-8. Br J Dermatol. 1998 Dec;139(6):1052–4. doi: 10.1046/j.1365-2133.1998.02564.x. [DOI] [PubMed] [Google Scholar]
- 121.Krown SE, Li P, Von Roenn JH, Paredes J, Huang J, Testa MA. Efficacy of low-dose interferon with antiretroviral therapy in Kaposi’s sarcoma: a randomized phase II AIDS clinical trials group study. J Interferon Cytokine Res. 2002 Mar;22(3):295–303. doi: 10.1089/107999002753675712. [DOI] [PubMed] [Google Scholar]
- 122.Yarchoan R, Pluda JM, Wyvill KM, Aleman K, Rodriguez-Chavez IR, Tosato G, et al. Treatment of AIDS-related Kaposi’s sarcoma with interleukin-12: rationale and preliminary evidence of clinical activity. Crit Rev Immunol. 2007;27(5):401–14. doi: 10.1615/critrevimmunol.v27.i5.10. [DOI] [PubMed] [Google Scholar]
- 123.Maio M. Melanoma as a model tumour for immuno-oncology. Ann Oncol. 2012 Sep;23(Suppl 8):viii10–4. doi: 10.1093/annonc/mds257. [DOI] [PubMed] [Google Scholar]
- 124.Gregory SM, West JA, Dillon PJ, Hilscher C, Dittmer DP, Damania B. Toll-like receptor signaling controls reactivation of KSHV from latency. Proc Natl Acad Sci U S A. 2009 Jul 14;106(28):11725–30. doi: 10.1073/pnas.0905316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.West JA, Gregory SM, Sivaraman V, Su L, Damania B. Activation of plasmacytoid dendritic cells by Kaposi’s sarcoma-associated herpesvirus. J Virol. 2011 Jan;85(2):895–904. doi: 10.1128/JVI.01007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fairley JL, Denham I, Yoganathan S, Read TR. Topical imiquimod 5% as a treatment for localized genital Kaposi’s sarcoma in an HIV-negative man: a case report. Int J STD AIDS. 2012 Dec;23(12):907–8. doi: 10.1258/ijsa.2012.012074. [DOI] [PubMed] [Google Scholar]
- 127.Lebari D, Gohil J, Patnaik L, Wasef W. Isolated penile Kaposi’s sarcoma in a HIV-positive patient stable on treatment for three years. Int J STD AIDS. 2014 Jul;25(8):607–10. doi: 10.1177/0956462413517494. [DOI] [PubMed] [Google Scholar]
- 128.Prinz Vavricka BM, Hofbauer GF, Dummer R, French LE, Kempf W. Topical treatment of cutaneous Kaposi sarcoma with imiquimod 5% in renal-transplant recipients: a clinicopathological observation. Clin Exp Dermatol. 2012 Aug;37(6):620–5. doi: 10.1111/j.1365-2230.2011.04278.x. [DOI] [PubMed] [Google Scholar]
- 129.Pourcher V, Desnoyer A, Assoumou L, Lebbe C, Curjol A, Marcelin AG, et al. Phase II Trial of Lenalidomide in HIV-Infected Patients with Previously Treated Kaposi’s Sarcoma: Results of the ANRS 154 Lenakap Trial. AIDS Res Hum Retroviruses. 2017 Jan;33(1):1–10. doi: 10.1089/AID.2016.0069. [DOI] [PubMed] [Google Scholar]
- 130.Steff M, Joly V, Di Lucca J, Feldman J, Burg S, Sarda-Mantel L, et al. Clinical activity of lenalidomide in visceral human immunodeficiency virus-related Kaposi sarcoma. JAMA Dermatol. 2013 Nov;149(11):1319–22. doi: 10.1001/jamadermatol.2013.5751. [DOI] [PubMed] [Google Scholar]
- 131.Rubegni P, Sbano P, De Aloe G, Flori ML, Fimiani M. Thalidomide in the treatment of Kaposi’s sarcoma. Dermatology. 2007;215(3):240–4. doi: 10.1159/000106583. [DOI] [PubMed] [Google Scholar]
- 132.Polizzotto MN, Uldrick TS, Wyvill KM, Aleman K, Peer CJ, Bevans M, et al. Pomalidomide for Symptomatic Kaposi’s Sarcoma in People With and Without HIV Infection: A Phase I/II Study. J Clin Oncol. 2016 Dec;34(34):4125–31. doi: 10.1200/JCO.2016.69.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016 Oct;17(10):1374–85. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016 Jun 30;374(26):2542–52. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015 Jan 22;372(4):320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 136.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015 Jul 2;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Foreman KE, Wrone-Smith T, Krueger AE, Nickoloff BJ. Expression of costimulatory molecules CD80 and/or CD86 by a Kaposi’s sarcoma tumor cell line induces differential T-cell activation and proliferation. Clin Immunol. 1999 Jun;91(3):345–53. doi: 10.1006/clim.1999.4712. [DOI] [PubMed] [Google Scholar]
- 138.Paydas S, Bagir EK, Deveci MA, Gonlusen G. Clinical and prognostic significance of PD-1 and PD-L1 expression in sarcomas. Med Oncol. 2016 Aug;33(8):93. doi: 10.1007/s12032-016-0807-z. [DOI] [PubMed] [Google Scholar]
- 139.Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013 Jul 1;19(13):3462–73. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]