Abstract
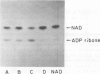
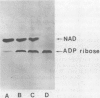
The quantity of membrane-bound and extracellular exotoxin A in four strains of Pseudomonas aeruginosa was investigated. In strain PA-103, which is the prototype strain used for toxin production, all of the toxin was released into the growth medium and little toxin remained with the cell envelope. In a virulent strain from a clinical source, strain 119, exotoxin A was found in equal amounts in the growth medium and in the cell envelopes. An avirulent mutant of this strain, strain 119 (AP-), which was resistant to 800 microgram of polymyxin B per ml, had all the exotoxin A in a membrane-bound state and was unable to release exotoxin A into the growth medium. Thus, exotoxin A can be found in the membrane-bound form, in the extracellular form, or in both. The quantities of membrane-bound toxin and extracellular toxin vary with the strain P. aeruginosa. The polymyxin B-resistant mutant which is blocked in toxin release will be useful in studies of exotoxin A secretion.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. R., Watkins W. M. Low magnesium and phospholipid content of cell wals of Pseudomonas aeruginosa resistant to polymyxin. Nature. 1970 Sep 26;227(5265):1360–1361. doi: 10.1038/2271360a0. [DOI] [PubMed] [Google Scholar]
- Chung D. W., Collier R. J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrer E., Teuber M. Induction of polymyxin resistance in Pseudomonas fluorescens by phosphate limitation. Arch Microbiol. 1977 Jul 26;114(1):87–89. doi: 10.1007/BF00429636. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Bayer M. E. Membrane-bound enterotoxin of Vibrio cholerae. J Gen Microbiol. 1977 Dec;103(2):381–387. doi: 10.1099/00221287-103-2-381. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Welsh K. M., Bayer M. E. Characterization of membrane-bound nicotinamide adenine dinucleotide glycohydrolase of Vibrio cholerae. J Biol Chem. 1979 Sep 25;254(18):9254–9261. [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Lyle R. D. Chemical alterations in cell envelopes of polymyxin-resistant Pseudomonas aeruginosa isolates. J Bacteriol. 1979 Jun;138(3):839–845. doi: 10.1128/jb.138.3.839-845.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Liu P. V., Kabat D. Mechanism of action of Pseudomonas aeruginosa exotoxin Aiadenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect Immun. 1977 Jan;15(1):138–144. doi: 10.1128/iai.15.1.138-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Sadoff J., Bjorn M. J., Maxwell E. S. Pseudomonas aeruginosa exoenzyme S: an adenosine diphosphate ribosyltransferase distinct from toxin A. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3211–3215. doi: 10.1073/pnas.75.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu P. V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973 Oct;128(4):506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Liu P. V., Yoshii S., Hsieh H. Exotoxins of Pseudomonas aeruginosa. II. Concentration, purification, and characterization of exotoxin A. J Infect Dis. 1973 Oct;128(4):514–519. doi: 10.1093/infdis/128.4.514. [DOI] [PubMed] [Google Scholar]
- MORIHARA K. PRODUCTION OF ELASTASE AND PROTEINASE BY PSEUDOMONAS AERUGINOSA. J Bacteriol. 1964 Sep;88:745–757. doi: 10.1128/jb.88.3.745-757.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H. Substrate specificity of elastolytic and nonelastolytic proteinases from Pseudomonas aeruginosa. Arch Biochem Biophys. 1966 Apr;114(1):158–165. doi: 10.1016/0003-9861(66)90317-1. [DOI] [PubMed] [Google Scholar]
- Moss J., Manganiello V. C., Vaughan M. Hydrolysis of nicotinamide adenine dinucleotide by choleragen and its A protomer: possible role in the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4424–4427. doi: 10.1073/pnas.73.12.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovskis O. R., Wretlind B. Assessment of protease (elastase) as a Pseudomonas aeruginosa virulence factor in experimental mouse burn infection. Infect Immun. 1979 Apr;24(1):181–187. doi: 10.1128/iai.24.1.181-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L., Kabat D., Iglewski B. H. Structure-activity relationships of an exotoxin of Pseudomonas aeruginosa. Infect Immun. 1977 Apr;16(1):353–361. doi: 10.1128/iai.16.1.353-361.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]