Abstract
Background
CD31, expressed by the majority of the neonatal T cell pool, is involved in modulation of T-cell Receptor signalling by increasing the threshold for T cell activation. Therefore, CD31 could modulate neonatal tolerance and adaptive immune responses.
Methods
Lymphocytes were harvested from murine neonates at different ages, human late preterm and term cord blood, and adult peripheral blood. Human samples were activated over a five-day period to simulate acute inflammation. Mice were infected with influenza; lungs and spleens were harvested at days 6 and 9 post-infection and analyzed by flow cytometry.
Results
CD31 expressing neonatal murine CD4+ and CD8a+ T cells increase over the first week of life. Upon in vitro stimulation, human infants’ CD4+ and CD8a+ T cells shed CD31 faster in comparison to adults. In the context of acute infection, mice infected at 3-days old have an increased number of naive and activated CD31+ T lymphocytes at the site of infection at day 6 and 9 post-infection, as compared to 7-days old; however, the opposite is true in the periphery.
Conclusion
Differences in trafficking of CD31+ Cytotoxic T Lymphocytes (CTLs) during acute influenza infection could modulate tolerance and contribute to a dampened adaptive immune response in neonates.
Introduction
The overall rate of preterm birth (infants <37 weeks gestation) was 9.6% and the rate of late preterm birth (infants 33–36 weeks gestation) was 6.9% in 2015 in the United States (1), which translates to 72% of all preterm births occurring in the late preterm period. Late preterm infants have significantly higher risk for respiratory disease and infections, which contributes to the use of twice as many healthcare dollars over the first 2 years of life, as compared to their term counterparts (2). The neonatal immune system is frequently cited as a culprit for neonatal susceptibility to infection; however, there is inconsistency in the literature about what constitutes an ideal term and preterm neonatal model in order to determine the specific mechanisms of susceptibility (3–6). Therefore, there is a real need to develop clinically relevant neonatal animal models of infection.
To better understand the development of the neonatal immune system, we have established a neonatal mouse model of influenza infection to determine the phenotypic and functional characteristics of both the innate and adaptive immune system, and to dissect the developmental mechanisms which regulate immature immune systems. In the context of an influenza infection, 3-day old mice respond less rapidly compared to adult or 7-day old mice (7). While they are able to generate a virus-specific response, the expansion of cytotoxic T lymphocytes (CTLs) is significantly slower which results in an increased rate of morbidity and mortality.
CD31 is a member of the immunoglobulin (Ig) superfamily of cell adhesion molecules. It is expressed on most cells of the hematopoietic lineage including platelets, monocytes, neutrophils, and T cells and plays an important role in the inflammatory response through the modulation of leukocyte activation, cytokine production and the maintenance of vascular barrier integrity (8–10). CD31 is involved in modulation of TCR-signalling. Engagement of CD31 on the surface of T cells reduces Zap70 phosphorylation through the action of protein tyrosine phosphatases such as SHP-2, which are recruited following phosphorylation of the two cytoplasmic tails of CD31 containing immunoreceptor tyrosine-based inhibitory motifs (ITIMs) (8,9,11). Following TCR signalling, CD31 engagement also leads to the inhibition of Jun-N-Terminal kinase (JNK), NF-κB, and IRF-3 activation, thereby increasing the threshold required for T cell activation (9).
CD31 plays a critical role in the regulation of the sensitivity of the T cell receptor. Recently, it was demonstrated that early fetal development supports loss of the regulatory co-receptor CD31 (12), and that this loss of CD31 potentially contributes to the extremely preterm infant’s immune dysregulation. Therefore, we questioned whether differential expression of CD31 on CD4+ and CTLs could contribute to a defective adaptive immune response in the murine neonate when infected with influenza. We compared this in vivo infection model with expression of CD31 in late preterm and term infants’ T lymphocytes, to determine similarities and differences in CD31 development between the human and the mouse.
Methods
Mice and infections
C57Bl/6 neonatal mice were generated using standard breeding procedures and 8 week old adult C57Bl/6 mice were purchased from Charles River Laboratory. The mice were housed under specific-pathogen-free conditions in an American Association for the Accreditation of Laboratory Animal Care-certified barrier facility at the Drexel University College of Medicine Queen Lane Campus animal facility. Animal work was carried out according to approved Institutional Animal Care and Use Committee protocols.
Neonatal mice at 3 days of age (weight ~3g) were infected intranasally (i.n.) with 0.12 TCID50 (0.04 TCID50/g) of influenza virus H1N1 strain PR8 (A/Puerto Rico/8/34) (generous gift of Dr. W. Gerhard, Wistar Institute, Philadelphia, PA) in a 5μl volume. Neonatal mice at 7 days of age (weight ~5g) were infected intranasally (i.n.) with 0.20 TCID50 (0.04 TCID50 /g) of influenza virus in a 7μl volume. Adult 8 week old C57Bl/6 mice (weight ~20g) were infected i.n. with a sublethal dose of 3 TCID50 in a 20μl volume (0.15 TCID50 /g). The mice were anesthetized with inhaled isoflurane before intranasal inoculations. The primary response was examined by harvesting cells from lungs and spleens on noted days post-infection.
Isolation of pulmonary lymphocytes
Pulmonary lymphocytes were isolated from individual mice by removing lungs and mincing into smaller pieces. The tissue was then digested for 2 h at 37°C with 3.0 mg/ml collagenase A and 0.15 μg/ml DNase I (Roche) in RPMI 1640 (Corning) containing 5% heat-inactivated FBS (Gemini), 2 mM l-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin (Corning). The digested tissue was then run through a 40-μm cell strainer (Falcon) and washed in the same media as above. Cells were counted using trypan blue exclusion with light microscopy.
All absolute cell numbers are calculated per 100 mg of lung tissue. Cells were fixed in 1% paraformaldehyde (Fisher Scientific) before flow cytometric analysis. Data was collected on a FACS LSR Fortessa using FACS Diva software (BD Biosciences). Analysis was performed using Flow Jo software (Tree Star).
Cord Blood collection
This study was approved by the Institutional Review Board of Drexel University College of Medicine. There was a waiver of documentation of written consent. Those patients with known major fetal anomalies, fetal demise, maternal active HSV infection, and mothers with known HIV infection were excluded. Cord blood sampling kits were preassembled to standardize collection procedures, ensuring uniform reagents were used (13). Umbilical cord blood was collected between February 2016 and October 2016, using universal precautions from 10 elective, full term cesarean section deliveries (38–40 weeks gestation), with no rupture of membranes or labor. In addition, samples were collected from 10 late preterm deliveries (33 to 36 weeks gestation), which were performed for maternal indications (preclampsia and/or uncontrolled hypertension). Peripheral venous blood was also obtained from five control healthy, nonsmoking adults (aged 20–40 y; median age 24 y). Study data were collected and managed using REDCap electronic data capture tools hosted at Drexel University (14). REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies. All specimens and data were de-identified. Cord blood was collected and stored at room temperature until processing within 24 hours of collection at the Drexel University College of Medicine, Department of Microbiology and Immunology.
Umbilical Cord Mononuclear Cell Isolation
All processing was completed using BSL 2 procedures. Prior to cell isolation, a cord blood aliquot was diluted with one part 10% RPMI to one part whole blood in a sterile 50 mL conical tube. Diluted blood was layered over Ficoll-Paque® (Lymphocyte Separation Medium, Cellgro) at a ratio of 3-mL blood to 1-mL separation medium. Blood was pipetted slowly down the side of the 50 mL conical to overlay the Ficoll-Paque. Samples were centrifuged at 20°C for 30 min at 900g with no brake. After centrifugation the mononuclear cell layer was removed and transferred to a new 50mL conical. The tube was filled with 5% RPMI and centrifuged for 5 minutes at 500g at 20°C, with the brake. Cells were washed with 5% RPMI, and then resuspended in 10% RPMI. Cells were frozen in 10×106 cells/mL aliquots in freeze media, 10% DMSO (Sigma) and 90% sterile-filtered prescreened FBS (Gemini Bioproducts). Cells were kept in long term storage in −150 degree freezers.
Human T cell isolation and activation
Cells were quick thawed from freezing and total T lymphocytes were isolated using EasySep Human T cell Enrichment Kit (STEMCELL Technologies). Purity was assessed by flow cytometry and was consistently greater the 90%. Cells were then incubated at 37°C in 10% RPMI containing 50U/mL recombinant Human IL-2 (Peprotech) and CD3/CD28 Dynabeads at a ratio of 3 beads per cell (Gibco). Two days post activation, cells were spun down and fresh media was added containing 50U/mL rHIL-2. Cells were harvested 5 days post activation, Dynabeads were removed and cells were analyzed by flow cytometry.
Flow cytometry
For mouse experiments, cells were co-stained with anti-mouse CD8α conjugated to PE (Tonbo Bioscience), anti-mouse CD4 conjugated to violetFluor450 (Tonbo Bioscience), anti-mouse CD44 conjugated to BV605 (Biolegend), anti-mouse CD62L conjugated to APC (eBioscience), and anti-mouse CD31 conjugated to PE-Cy7 (eBioscience). All stains were completed on ice to prevent internalization. For human studies, cells were co-stained with anti-human CD8α conjugated to APC (eBioscience), anti-human CD4 conjugated to PerCP-Cy5.5 (eBioscience), anti-human CD45RO conjugated to APC-efluor780 (eBioscience), anti-human CD45RA conjugated to evolve605 (eBioscience), and anti-human CD31 conjugated to PE (eBioscience). All stains were completed on ice to prevent internalization. Cells were fixed in 1% paraformaldehyde (Fisher Scientific) before flow cytometric analysis. Data was collected on a FACS LSR Fortessa using FACS Diva software (BD Biosciences). Analysis was performed using Flow Jo software (Tree Star). For mouse and human studies, lymphocytes were gated based on forward and side scatter, and then by CD4+ or CD8a+. In the mouse, naïve cells were gated based on expression of CD62L+CD44− and activated cells were gated by CD44+CD62L−. In the human, naïve cells were gated by CD45RA+CD45RO− expression, while activated cells were gated by CD45RO+CD45RA− expression.
Statistical analysis
Statistical analysis was performed using the nonparametric Mann Whitney test for unpaired samples. Analyses were performed using GraphPad PRISM version 5 for Mac (GraphPad Software, San Diego California, www.graphpad.com). P values<0.05 were considered to be statistically significant.
Results
Characterization of Naive CD31+CD8a+ and CD31+CD4+ T cells in the murine neonate
We first sought to determine the developmental regulation of CD31 expression on CD8a+ and CD4+ T cells in the neonatal mouse. Spleens were harvested from mice at 1, 3, 5 and 7 days of life (DOL) and compared to adult mice at 8 weeks of age. Studies in the mouse have indicated that the first three weeks of a T cell’s residency in the lymphoid periphery constitute a transition period for Recent Thymic Emigrants (RTEs) (15,16) and so we questioned how expression of CD31+ would change on naïve T cells after their egress from the thymus. Naïve T lymphocytes were identified by CD62L+ cell surface expression. Frequencies of naïve CD31+ T lymphocytes (CD8a+CD62L+CD31+ and CD4+CD62L+CD31+) were determined (Figure 1a–b). Two distinct populations were identified based on differential expression of CD31 (noted here out as CD31hi and CD31int) in both CD4+ and CD8a+ populations (Figure 1c). No significant differences were identified in the frequency of CD4+CD62L+ CD31hi or CD31int expressing cells across the first week of life (Figure 1a). CD8a+ T cells however, display a decrease in the frequency of CD31int T cells over time (65% (DOL1) to 21% (DOL7) and 10% (adults); p<0.05 all time points relative to DOL 1)) (Figure 1b). Next, we wished to compare the absolute number of CD31+ cells. In the first week of life, CD4+CD31hi lymphocytes make up the majority of the CD4+ T cell pool, and increase significantly during development (4×103 (DOL1) to 4×105 (DOL7); p<0.05 for D1 to all other time points and D3 to D5), while CD4+CD31int remain comparatively stable (Figure 1d). CD8a+CD31hi T cells also increase over development, with a 10 fold increase in the absolute number from the first day of life to the seventh day of life (1.6×104 (DOL1) to 1.4×105 (DOL7); p<0.05 for D3 to D5 and D5 to D7) (Figure 1e). This is consistent with previous work in humans, which demonstrated an increase in CD31 expression across gestation (12).
Figure 1. Comparison of CD31+ T lymphocytes in the neonatal and adult mouse.
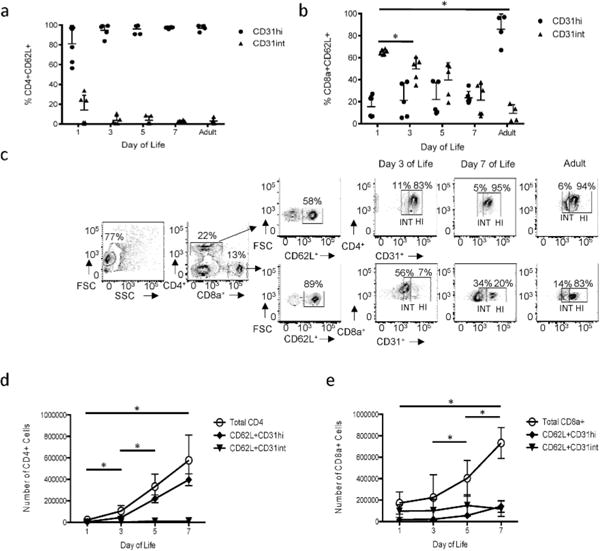
Spleens were harvested at noted day of life and analyzed by flow cytometry. Frequency of CD31hi and CD31int (a) CD4+ T cells and (b) CD8a+ T cells. Each dot represents a single animal. (n=4–6 animals per group, three independent experiments) (p<0.05 for CD31int). (c) Representative flow plots depicting the gating strategy of CD31hi and CD31int designations for CD4+ (Top panel) and CD8a+ (Bottom panel) T lymphocytes at noted day of life. Absolute number of naïve (d) CD4+ CD31hi and CD31int expressing cells relative to the total CD4+ population and (e) CD8a+ CD31hi and CD31int expressing cells relative to the total CD8a+ population (P<0.05 for CD31hi). Nonparametric (Mann-Whitney) statistics were used to compare expression level/day of life.
CD31 frequency in human late preterm and term cord blood
Next, we sought to determine differences in expression of CD31 on naïve lymphocytes in late preterm cord blood samples. Cord blood samples were used from late preterm infants (33–36 weeks) where there had been a maternal indication for premature delivery, specifically preeclampsia or hypertension. Term samples (38–40 weeks) were obtained from scheduled and elective cesarean sections, without spontaneous labor. Healthy human adult (20–40 years of age) controls were used as a comparison for analysis of CD31 expression on CD4+ and CD8a+ T lymphocytes. Naïve cells were identified by CD45RA+CD45RO− cell surface expression (Supplemental Figure S1 (online)). In agreement with our mouse studies, two distinct populations of expression, CD31high and CD31intermediate, were identified in both CD4+ and CD8a+ T cells. Also similar to the mouse, there are no significant differences in the frequency of CD4+ CD45RA+CD31high or intermediate cells during development (Figure 2a and b). In addition, CD8a+ CD45RA+CD31hi lymphocyte frequency remains stable with age (Figure 2d and e).
Figure 2. CD31 expression on naive and activated Late Preterm, Term and Adult T cells.
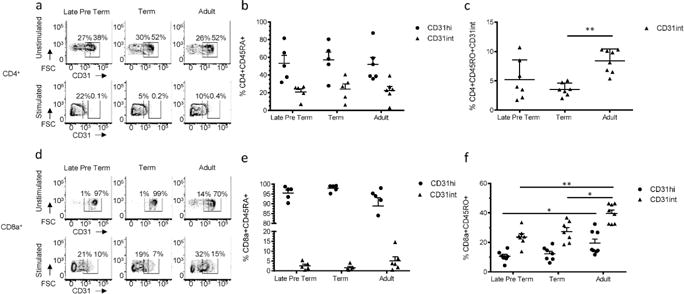
Total T lymphocytes were isolated from Late Preterm, Term, and Adult samples. Cells were stained as naïve or stained after 5 days of stimulation and expansion. Representative flow plots of CD31 gating strategy of CD31hi and CD31int for unstimulated (Top Row) or stimulated (Bottom Row) (a) CD4+ T cells and (d) CD8a+ T cells. Frequency of unstimulated naïve CD45RA+CD45RO− CD31 hi or CD31int (b) CD4+ or (e) CD8a+ T cells. Each dot represents a single patient sample (n=5–6 samples per group, three independent experiments). Frequency of cells activated with CD3/CD28 stimulation in the presence of IL-2 CD45RO+CD45RA− (c) CD4+ CD31int T cells (p<0.005) and (f) CD8a+ CD31hi and int T cells (late preterm to adult CD31int p<0.005; late preterm to adult CD31hi p<0.05; term to adult CD31hi p<0.05) (n=4–6 samples per group, two independent experiments). Nonparametric (Mann-Whitney) statistics were used to compare expression level/day of life.
Characterization of CD31 expression in human T cells upon stimulation
CD31 plays an important role in the regulation of TCR signaling. We questioned how expression of CD31 would change in preterm and term samples in a pro-inflammatory environment, such as in the setting of prolonged rupture of membranes or chorioamnionitis. To simulate this environment, we used a 5 day in vitro stimulation. T lymphocytes isolated from the same samples used for the naïve expression described above were activated by CD3/CD28 stimulation and expanded in the presence of IL-2. Following expansion, all activated T cells, identified by CD45RO+CD45RA− cell surface expression, display a dramatic decrease in the expression of CD31+ (Figure 2a, c, d and f). After activation, the CD31hiCD4+ population dramatically decreases to almost undetectable levels (data not shown) and the frequency of CD31intCD4+ cells is also greatly reduced, regardless of age, consistent with previous work (17,18). There are no statistically significant differences in the frequencies of activated CD4+ CD31+ T cells between late preterm and term infants (Figure 2c). However, when term infants are compared to adults, there is a significant difference in the frequency of activated CD31int in term samples (4% and 9% for Term and Adults, respectively; p<0.005), which indicates a more rapid shedding of CD31 from CD4+ T cells in term infants upon activation. In addition, activated late preterm and term CD8a+ lymphocytes demonstrate an increase in CD31 shedding (Figure 2f), as compared to their adult counterparts (CD31hi 11%, 12% and 20% for Late Pre-term, Term and Adult respectively; p<0.05 Late Pre-Term to Adult; CD31int 24%, 27%, and 40% for Late Pre-term, Term and Adult respectively; p<0.005 Late Pre-term to adult, p<0.05 Term to Adult).
Differences in CD31 expression at the site of infection and in the periphery in the murine neonate
Given the increased shedding of CD31 from CD8+ T cells in human newborn samples upon in vitro stimulation, we wanted to explore the impact of an in vivo acute infection on CD31 expression using our neonatal murine model. To pursue this question, mice were infected intranasally with influenza virus at 3-days of life, 7-days of life, and 8-weeks old; lungs were harvested at 6 and 9 days post-infection. Samples were normalized per 100mg of lung given the difference in lung size between the neonate and adult mouse. Interestingly, when mice are infected on day 3 of life, there is a ten-fold increase in the number of CD4+ and CD8+ CD31+ T lymphocytes in the lungs of animals at 6 days post-infection, as compared to the animal infected on day 7 of life or the adult (p<0.005 DOL3 to DOL7, p<0.05 DOL3 to Adult) (Figure 3a). However, by day 9 post-infection there is no statistical difference between different aged animals (Figure 3d). To determine which specific cells were expressing high levels of CD31, we compared the absolute numbers of pulmonary CD31hi activated (CD44+CD62L−) and naïve (CD62L+CD44−) cells. Surprisingly, animals infected at 3-days of life display a five-fold increase in the number of naïve CD31hi CD4+ and CD8a+ T cells in the lung at 6 days post-infection (p<0.05 for both CD4+ and CD8a+) (Figure 3 b and c). By 9 days post-infection, no significant difference is detectable in the number of naïve CD4+CD31hi cells, although there are differences in activated CD4+CD31hi cells (2.5×105 (DOL3) and 1.6×105 (DOL7); p<0.05) (Figure 3e). Contrary to the CD4+ population, CD8a+ T cells continue to demonstrate increased numbers of both CD31hi naïve and activated cells at day 9 post-infection (CD8a+CD31hiCD62L+: 2.6×105 (DOL3) and 1.22×105 (DOL7);p<0.005, CD8a+CD31hiCD44+: 2×105 (DOL3) and 3.3×104 (DOL7); p<0.0001) (Figure 3 f and j).
Figure 3. Differences in CD31 expression at the site of infection and in the periphery in the murine neonate.
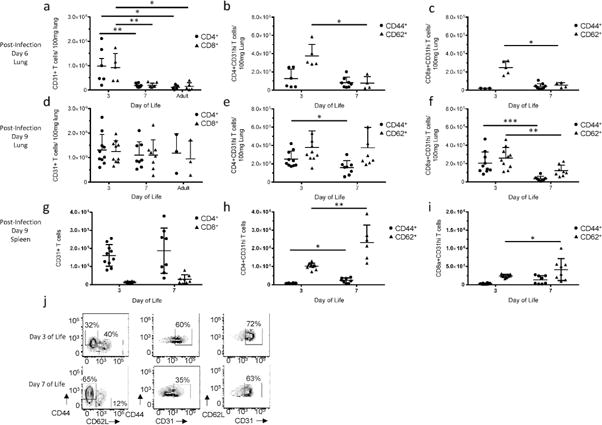
Mice were infected intranasally with Influenza type A on noted day of life. Lungs were then harvested and processed for analysis by flow cytometry. CD31+ CD4+ and CD8a+ absolute numbers were calculated per 100mg of lung at (a) day 6 post-infection (CD4 and CD8a D3 to D7p<0.005; D3 to Adult p<0.05) and (d) day 9 post-infection. Each dot represents a single animal (n=3–10 animals per group, three independent experiments). Cells were then analyzed for cell surface expression of a naïve (CD62L+CD44−) or activated (CD44+CD62L−) phenotype. Absolute numbers per 100mg of lung were calculated for (b) CD4+CD31hi cells (p<0.05) and (c) CD8a+ CD31hi cells at day 6 post-infection (p<0.05). Absolute numbers per 100mg of lung were also calculated for (e) CD4+CD31hi cells (p<0.05) and (f) CD8a+ CD31hi cells at day 9 post-infection (Naïve p<0.005, activated p<0.0001). (g) Total splenic CD31+ CD4+ and CD8a+ T lymphocytes at day 9 post-infection. Naïve and activated splenic (h) CD31hi CD4+ (naïve p<0.005, activated p<0.05) and (i) CD8a+ (naïve p<0.05) cells at day 9 post infection. (j) Representative flow plots depicting the evolution of CD31 expression in naïve and activated CD8a+ T cells at day 9 post-infection in the lung between animals infected at Day 3 of life (Top Row) and Day 7 of Life (Bottom Row). Nonparametric (Mann-Whitney) statistics were used to compare expression level/day of life.
Next, we questioned whether there would be similar differences in CD31 expression on T lymphocytes in a peripheral lymphoid organ, such as the spleen, in the context of acute infection. At 9 days post-infection, there is not a significant difference in the number of total CD31+ T lymphocytes between mice infected at 3 and 7 days of age (Figure 3g). However, in direct contrast to the findings at the site of infection, there are more activated and naïve CD4+CD31hi T cells (Figure 3h) and naïve CD8a+CD31hi T cells (Figure 3i) in the spleen of the animal infected at 7 days of life as compared to the 3-day old. Together, this data from the lung and spleen indicate a role for CD31 in trafficking in the neonatal mouse. We have previously demonstrated a defective adaptive immune response to influenza in the neonatal mouse, and a major contributing factor is the TCR (7). Increased expression of a known TCR inhibitor at the site of infection could be contributing to a dampened CTL response to acute viral infection.
Discussion
The neonate must be tolerant to both maternal alloantigens as a fetus, as well as the sudden enormous exposure to environmental antigens after birth. The exact mechanisms which regulate this tolerance have not been determined. In humans, CD31 is expressed on recent thymic emigrants (RTEs), but lost during postthymic peripheral expansion of RTEs (19), which leads to a decrease in naive CD4+ and CD8+ T cells which express CD31 during ageing from childhood to adulthood. T cells likely use CD31 for transendothelial migration to enter secondary lymphoid organs, a process which is essential for homeostatic proliferation (20). T-cells in the periphery require self-MHC/TCR interactions to act as a survival signal and promote homeostatic proliferation (21). CD31 is lost during in vitro stimulation of CD4+ cells after TCR triggering (17). Therefore, one potential explanation for down-regulation of CD31 expression on peripherally expanded naive T cells could be a consequence of TCR ligation with endogenous MHC and self-peptides. CD31 expression could play a role in the maintenance of neonatal T cell quiescence and neonatal tolerance given that CD31-deficient mice demonstrate a resistance to the development of HY-mismatched skin graft induced T-cell tolerance following the delivery of antigenic peptide intranasally (8).
Therefore, we wanted to establish the development of the recent thymic emigrant (RTE) marker, CD31, in the mouse and human and its possible role in modulating T cells at different ages in response to infection in the mouse. The biological significance of CD31, particularly in the late preterm and term neonate, has not been fully elucidated. Here, we demonstrate that there is an increase in the absolute numbers of CD4+CD31+ T cells and CD8+CD31+ T cells over the first week of life in the murine neonate. This is in agreement with previous work which demonstrated an increase in the frequency of CD31+ T cells over the course of human gestation(12), which verifies an important connection between our neonatal murine model and the late preterm human neonate.
Maintenance of the peripheral naive T cell compartment is primarily dependent on thymic output in mice (22) and young humans (23), although this changes with aging in humans (22,24). Naïve CD4+ T cells which express CD31 contain elevated T cell Receptor Excision Circles (TREC), and are therefore believed to have arisen from thymic production (25). The mouse neonate is comparably more lymphopenic than the human neonate. At birth, CD8+ T cells are outnumbered by CD4+ T cells approximately 2:1 with CD8+ T cells making up only around 0.5% of the total lymphocyte pool, which shifts with a significant efflux of lymphocytes from the thymus of murine neonates, particularly after the third day of life (26). Consistent with this, our data demonstrates an increase in the absolute number of CD31hi expressing CD4+ and CD8+ lymphocytes over the first week of life. As a comparison to the mouse, in the setting of hematopoietic stem cell transplantation and resultant lymphopenia, CD31 has been shown to be a reliable marker of reemerging naïve CD4+ T cells in humans (24,27)
Shedding of CD31 is observed upon lymphocyte activation and differentiation (17), especially in human CD4+ T cells activated via TCR stimulation (28). Upon cell activation, the loss of T cell CD31 is the result of its cleavage and shedding into biologic fluids, which then results in the loss of its inhibitory function, as the necessary trans-homophilic engagement of the molecule cannot be established (18). Here, we demonstrate upon TCR stimulation with CD3 and CD28, there is an increase in shedding of CD31 from neonatal CD4+ and CD8+ T cells, as compared to adults. However, there was not a difference between late preterm and term samples. One possible explanation is an insufficient sample size. Another possible explanation is that specific TCR stimulation may be saturating the T cells and be forcing an unnatural decrease in expression in an in vitro experiment. Given the difficulty associated with the collection of PBMCs from acutely infected human newborns patients, it is a challenge to determine changes in CD31 expression in the setting of human infection.
Around the time of birth, there is a massive efflux of RTEs from the thymus, and the immaturity of these cells contributes to infection susceptibility in preterm and term neonates. Neonates display delayed T cell function as a result of a greater proportion of naïve T cells in the circulation and a low subpopulation of memory T cells (29). In addition to the sheer numbers of RTEs, these naïve T cells are dependent on dendritic cells which have been shown to be inefficient in antigen uptake (30). The absolute number of CD31hiCTLs increases over the first week of murine life. However, when compared to the total CTL population, they comprise a relatively small percentage of the CTLs. Strikingly, in the setting of acute viral infection, CD31+CTLs comprise a significant number of the infiltrating CTLs at the site of infection in the 3-day old murine neonate at 6 days post-infection, compared to the 7-day old and adult murine animal. In addition, the CD8a+CD31+ cells of animals infected at day 3 of life are primarily naïve at the site of infection at both days 6 and 9 post-infection. Similarly, CD4+CD31+ cells comprise a large portion of cells within the lungs of animals infected at day 3 of life. This high level of CD31 expression on both naïve and activated CTLs is specific to the site of infection in the young neonate. In the spleen, there is much less CD31 expression in the 3-day old neonate demonstrating that the high CD31 expression seen in the lung is not reflective of a global difference in CD31 expression, but rather a site-specific difference. Together, these data indicate a selective pressure in neonatal mice to maintain a tolerant environment at the site of infection. CD31 signals protect the host under conditions of immunological stress; CD31-deficient mice display exaggerated disease severity in inducible experimental models of T-cell-mediated inflammation, including experimental autoimmune encephalomyelitis (31) and endotoxemia (32,33). In addition, CD31 protects the endothelium from immune-mediated damage (34). Therefore, CD31 might be playing a protective role in the setting of influenza infection in the neonate by trafficking “dampened” CTLs to the site of infection.
To our knowledge, this is the first description of the development of CD31 in the murine neonate, and the use of a neonatal animal model to show differential expression of CD31 in the setting of an acute viral infection. Further murine and human mechanistic studies which examine CTL function in the setting of acute infection are needed. The impact of CD31 expression in the preterm and term neonate during an acute infection, such as chorioamnionitis or early onset sepsis, needs to be further investigated.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the obstetrical physicians and nurses in the Labor and Delivery Unit at Hahnemann University Hospital for subject identification and sample collection.
Statement of Financial Support:
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K08AI108791 to AC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. In addition, funding was provided by the Margaret Q. Landenberger Foundation.
Footnotes
Disclosure:
The authors have no financial relationships pertaining to this article. The authors confirm that there is no potential, perceived, or real conflict of interest. No author has a proprietary interest.
References
- 1.Martin JA, Hamilton BE, Osterman MJ. Births in the United States, 2015. NCHS data brief. 2016:1–8. [PubMed] [Google Scholar]
- 2.Berard A, Le Tiec M, De Vera MA. Study of the costs and morbidities of late-preterm birth. Archives of disease in childhood Fetal and neonatal edition. 2012;97:F329–34. doi: 10.1136/fetalneonatal-2011-300969. [DOI] [PubMed] [Google Scholar]
- 3.Kamath AT, Rochat AF, Christensen D, et al. A liposome-based mycobacterial vaccine induces potent adult and neonatal multifunctional T cells through the exquisite targeting of dendritic cells. PLoS One. 2009;4:e5771. doi: 10.1371/journal.pone.0005771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lines JL, Hoskins S, Hollifield M, Cauley LS, Garvy BA. The migration of T cells in response to influenza virus is altered in neonatal mice. J Immunol. 2010;185:2980–8. doi: 10.4049/jimmunol.0903075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gareau MG, Wine E, Reardon C, Sherman PM. Probiotics prevent death caused by Citrobacter rodentium infection in neonatal mice. J Infect Dis. 2010;201:81–91. doi: 10.1086/648614. [DOI] [PubMed] [Google Scholar]
- 6.Yasui H, Kiyoshima J, Hori T. Reduction of influenza virus titer and protection against influenza virus infection in infant mice fed Lactobacillus casei Shirota. Clin Diagn Lab Immunol. 2004;11:675–9. doi: 10.1128/CDLI.11.4.675-679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carey AJ, Gracias DT, Thayer JL, et al. Rapid Evolution of the CD8+ TCR Repertoire in Neonatal Mice. Journal of immunology. 2016;196:2602–13. doi: 10.4049/jimmunol.1502126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma L, Mauro C, Cornish GH, et al. Ig gene-like molecule CD31 plays a nonredundant role in the regulation of T-cell immunity and tolerance. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:19461–6. doi: 10.1073/pnas.1011748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marelli-Berg FM, Clement M, Mauro C, Caligiuri G. An immunologist’s guide to CD31 function in T-cells. Journal of cell science. 2013;126:2343–52. doi: 10.1242/jcs.124099. [DOI] [PubMed] [Google Scholar]
- 10.Privratsky JR, Newman DK, Newman PJ. PECAM-1: conflicts of interest in inflammation. Life sciences. 2010;87:69–82. doi: 10.1016/j.lfs.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newton-Nash DK, Newman PJ. A new role for platelet-endothelial cell adhesion molecule-1 (CD31): inhibition of TCR-mediated signal transduction. Journal of immunology. 1999;163:682–8. [PubMed] [Google Scholar]
- 12.Scheible KM, Emo J, Yang H, et al. Developmentally determined reduction in CD31 during gestation is associated with CD8+ T cell effector differentiation in preterm infants. Clinical immunology. 2015;161:65–74. doi: 10.1016/j.clim.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheible K, Secor-Socha S, Wightman T, et al. Stability of T cell phenotype and functional assays following heparinized umbilical cord blood collection. Cytometry Part A: the journal of the International Society for Analytical Cytology. 2012;81:937–49. doi: 10.1002/cyto.a.22203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berzins SP, Boyd RL, Miller JF. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. The Journal of experimental medicine. 1998;187:1839–48. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berzins SP, Godfrey DI, Miller JF, Boyd RL. A central role for thymic emigrants in peripheral T cell homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9787–91. doi: 10.1073/pnas.96.17.9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demeure CE, Byun DG, Yang LP, Vezzio N, Delespesse G. CD31 (PECAM-1) is a differentiation antigen lost during human CD4 T-cell maturation into Th1 or Th2 effector cells. Immunology. 1996;88:110–5. doi: 10.1046/j.1365-2567.1996.d01-652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fornasa G, Groyer E, Clement M, et al. TCR stimulation drives cleavage and shedding of the ITIM receptor CD31. Journal of immunology. 2010;184:5485–92. doi: 10.4049/jimmunol.0902219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimmig S, Przybylski GK, Schmidt CA, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. The Journal of experimental medicine. 2002;195:789–94. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dummer W, Ernst B, LeRoy E, Lee D, Surh C. Autologous regulation of naive T cell homeostasis within the T cell compartment. Journal of immunology. 2001;166:2460–8. doi: 10.4049/jimmunol.166.4.2460. [DOI] [PubMed] [Google Scholar]
- 21.Boyman O, Letourneau S, Krieg C, Sprent J. Homeostatic proliferation and survival of naive and memory T cells. European journal of immunology. 2009;39:2088–94. doi: 10.1002/eji.200939444. [DOI] [PubMed] [Google Scholar]
- 22.den Braber I, Mugwagwa T, Vrisekoop N, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–97. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Haddad R, Guimiot F, Six E, et al. Dynamics of thymus-colonizing cells during human development. Immunity. 2006;24:217–30. doi: 10.1016/j.immuni.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Junge S, Kloeckener-Gruissem B, Zufferey R, et al. Correlation between recent thymic emigrants and CD31+ (PECAM-1) CD4+ T cells in normal individuals during aging and in lymphopenic children. European journal of immunology. 2007;37:3270–80. doi: 10.1002/eji.200636976. [DOI] [PubMed] [Google Scholar]
- 25.Correa-Rocha R, Perez A, Lorente R, et al. Preterm neonates show marked leukopenia and lymphopenia that are associated with increased regulatory T-cell values and diminished IL-7. Pediatric research. 2012;71:590–7. doi: 10.1038/pr.2012.6. [DOI] [PubMed] [Google Scholar]
- 26.Kelly KA, Scollay R. Seeding of neonatal lymph nodes by T cells and identification of a novel population of CD3-CD4+ cells. European journal of immunology. 1992;22:329–34. doi: 10.1002/eji.1830220207. [DOI] [PubMed] [Google Scholar]
- 27.Muraro PA, Douek DC, Packer A, et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. The Journal of experimental medicine. 2005;201:805–16. doi: 10.1084/jem.20041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohler S, Thiel A. Life after the thymus: CD31+ and CD31-human naive CD4+ T-cell subsets. Blood. 2009;113:769–74. doi: 10.1182/blood-2008-02-139154. [DOI] [PubMed] [Google Scholar]
- 29.Walker JC, Smolders MA, Gemen EF, Antonius TA, Leuvenink J, de Vries E. Development of lymphocyte subpopulations in preterm infants. Scandinavian journal of immunology. 2011;73:53–8. doi: 10.1111/j.1365-3083.2010.02473.x. [DOI] [PubMed] [Google Scholar]
- 30.Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: prematurity means more than immaturity. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2011;24:25–31. doi: 10.3109/14767058.2010.482605. [DOI] [PubMed] [Google Scholar]
- 31.Graesser D, Solowiej A, Bruckner M, et al. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. The Journal of clinical investigation. 2002;109:383–92. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maas M, Stapleton M, Bergom C, Mattson DL, Newman DK, Newman PJ. Endothelial cell PECAM-1 confers protection against endotoxic shock. American journal of physiology Heart and circulatory physiology. 2005;288:H159–64. doi: 10.1152/ajpheart.00500.2004. [DOI] [PubMed] [Google Scholar]
- 33.Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. The American journal of pathology. 2005;166:185–96. doi: 10.1016/S0002-9440(10)62243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung K, Ma L, Wang G, et al. CD31 signals confer immune privilege to the vascular endothelium. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E5815–24. doi: 10.1073/pnas.1509627112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


