Abstract
Cancerous cells must cooperate with the surrounding stroma and non-malignant cells within the microenvironment to support the growth and invasion of the tumor. The nervous system is a component of every organ system of the body, and therefore, is invariably at the front line of the tumor invasion. Due to the complexity of the nervous system physiology, this review separately discusses the contributions of the central and peripheral nervous systems to the tumorigenesis and tumor progression. We further focus the discussion on the evidence that Schwann cells aid in tumor growth and invasion. Schwann cells, a largely unexplored element of the tumor microenvironment, may participate in the creation of tumor-favorable conditions through both bi-directional interaction with cancer cells and the facilitation of the immune-suppressive microenvironment through the mechanism of neural repair and immunomodulation.
Keywords: Tumor microenvironment, Schwann cell, Nervous system, Neuroinflammation, Regulatory myeloid suppressor cells
Introduction
It is now well established that cancer cells rely on the recruitment and interaction with various non-malignant cells to support tumor growth, creating what is referred to as the tumor microenvironment [1, 2]. Endothelial cells, fibroblasts and other stromal cells, as well as various immune cells play an important role in enabling or enhancing tumor capabilities [2]. Similar to the vasculature, neurons are found in virtually every organ system of the body and therefore serve as an early witness of the emerging tumor. The nervous system is not a simple bystander, however, and has also been extensively implicated in promoting tumor growth and progression. For simplicity, the discussion in this article is divided into two parts: the central nervous system (CNS), which includes the brain and the spinal cord, and the peripheral nervous system (PNS). PNS can be further subdivided into afferent neurons which sense various ques and send signals from the periphery to the CNS, and efferent neurons which control the motor and autonomic functions via the signaling from the CNS to the peripheral organs. Sympathetic and parasympathetic neurons comprise the visceral efferent system, controlling the involuntary activity of the body such as heart rate, respiration and perspiration.
In the last two decades, preclinical and clinical studies have established examples of the interaction between CNS as well as peripheral neurons and some tumors. We will review here several of these examples which implicate nervous system in playing either a direct role or indirectly influencing the tumor microenvironment.
Neuroglial cells (glia or neuroglia) are non-neuronal cells that provide support and protection for neurons through myelin formation and maintenance of homeostasis. In the CNS, glial cells include astrocytes, oligodendrocytes, ependymal cells and microglia. Schwann cells and satellite cells comprise the glia of the PNS. The glia of the CNS is known to contribute to the establishment of the microenvironment niche for brain metastasis through the production of pro-inflammatory mediators. The role of the peripheral glia in tumorigenesis and tumor progression is less defined. Schwann cells, which are best known for myelinating peripheral nerves, play an important role in neural regeneration, in part though the modulation of the immune system. In doing so, Schwann cells may be inadvertently aiding in the maintenance of microenvironment favorable to the tumor progression. We will review what is known currently about the interactions of tumors with Schwann cells, and provide additional perspective on the possible role Schwann cells play in modulating the tumor microenvironment.
Central nervous system
The potential bidirectional link between the brain and the peripheral malignant tumor has been the subject of multiple studies [3, 4]. Clinical imaging studies detect altered brain metabolism in patients with malignant disease [5–7]. Epidemiological studies indicate that psychosocial factors such as stress, depression and social isolation likely play a role in cancer progression [8–11]. These results indicate that CNS may sense and affect tumor progression, but while psychosocial interventions that teach stress management often provide positive effects on the quality of life [12], it remains controversial whether such interventions ultimately affect cancer progression and survival [13–16]. Nevertheless, accumulating evidence from clinical, in vitro and in vivo studies highlights specific stress response pathways which may influence cancer progression [17]. These stress response pathways directly affect well known “hallmarks of cancer” [1], including tumor proliferation and invasion, angiogenesis and evasion of the immune surveillance. The main signal transduction during the activation of the stress response occurs through the hypothalamic–pituitary–adrenal axis or the autonomic nervous system. The hypothalamic–pituitary–adrenal stress response results in downstream release of glucocorticoid hormones such as cortisol from the adrenal cortex [18]. Stress also modulates the levels of other neuroendocrine factors such as substance P, nerve growth factor, dopamine, oxytocin and prolactin [17, 19]. Patients with cancer exhibit alterations in diurnal serum cortisol rhythms [20], and flattening of such rhythms in patients with breast and lung cancer predicts early mortality [21, 22]. Several cancer cell types downregulate the expression of glucocorticoid receptor to escape glucocorticoid-induced apoptosis [23, 24]. Additional direct pro-tumorigenic effects of glucocorticoids may be through the compromise of DNA repair mechanism [25], or the suppression of cell-mediated immunity [26]. The stress response may, therefore, contribute to tumor progression, but it is still unknown whether the CNS receives signaling from the periphery about the presence of the tumor, and if so, how is that information processed and what responses are generated as a result?
Autonomic nervous system
The peripheral autonomic nervous system regulates gene expression of the cancer cells directly and elicits a wide influence on the tumor microenvironment [27]. The sympathetic nervous system (SNS) in particular plays a major role in tumorigenesis [27]. Practically, all organ systems in humans are regulated by the SNS via the catecholamine neurotransmitters either through the release of norepinephrine by the tissue localized nerve terminals or through the vascular distribution of epinephrine secreted by the adrenal gland. The influence of the SNS on tumor progression likely occurs not via an acute “fight-or-flight” stress response, but through sustained changes in basal levels and circadian cycles of SNS activity leading to durable alteration of the gene expression profile of cancer and supporting stroma elements [28–31]. Catecholamines released by the SNS bind adrenergic receptors which are expressed by most tissues and organ systems, including tumor cells and tumor stroma [32, 33]. The density of autonomic nervous fibers in prostate cancer and surrounding tissue is associated with poor clinical outcomes [34], and the link between SNS signaling and cancer is further supported by pharmaco-epidemiologic studies which suggest that β-adrenergic antagonists may reduce the progression of certain tumors [33, 35–38]. Chronic and circadian variations in the SNS activity alter hematopoietic environment, leading to a shift toward a pro-inflammatory pool of circulating leukocytes [39]. In addition, β-adrenergic signaling within tumor microenvironment may lead to the release of pro-inflammatory cytokines, chemotactic and pro-angiogenic factors, and matrix metalloproteinases, further aiding in tumor survival and progression [40–46]. Catecholamines may also lead to chromosomal instability and tumor initiation via direct effects of β-adrenergic signaling on tumor cells such as activation of Src and HER2 oncogenes [47, 48], inhibition of DNA damage repair and apoptosis [49, 50].
It is evident through the multitude of the epidemiologic and experimental body of work that stress response induced through the CNS driven hypothalamus–pituitary–adrenal axis and the sympathetic nervous system plays a significant part in the early stages of tumor progression via direct effects on the malignant cells and indirectly via aiding in the creation of tumor-favorable microenvironment.
Afferent nervous system
Compared with the SNS, much less is known about the role of the afferent (sensory) division of the PNS in tumor progression. Ablation of the sensory neurons in the mouse models of basal cell carcinoma and pancreatic ductal adenocarcinoma attenuates the initiation and progression of tumors [51, 52]. Thus, while this area of research is still in the early phase, sensory neurons likely make an important contribution to the tumor microenvironment. Most organs are innervated by the sensory neuron fibers through either the nodose (via the vagal nerve) or the spinal ganglia (via splanchnic nerves). The bulk of the cell soma of primary afferent neurons is located in the dorsal root ganglia (DRG) of the vertebral column. Pain, temperature, pressure, proprioception and other sensory information is carried by afferent neurons to the CNS. In response to tissue damage, injury or infection, DRG neurons are able to modulate the inflammatory response through the production of chemokines, cytokines and their associated receptors, including IL-1, IL-6, TNF-α, MCP-1, IP-10, CCR1 and CXCR4 to name a few [53–56]. Sensory neurons also produce neurotrophic factors, Toll-like receptors [53, 56, 57], and secrete histamine and glutamate as well as neuropeptides such as substance P, vasoactive intestinal polypeptide and calcitonin [58, 59]. Neurogenic inflammation has been primarily studied in the context of neurodegeneration, neuropathic pain and chronic pain syndrome [53, 56].
Most of the work on the relationship between afferent neurons and cancer focused on cancer-related pain syndrome and perineural invasion [60–62], two processes which are directly related to the pro-inflammatory activity of the sensory neurons and the upregulation of neurotrophic factors. The link between neurogenic inflammation and the development of cancer is well illustrated by the studies of the mouse models of pancreatitis and pancreatic cancer. Chronic pancreatic inflammation is a significant risk factor for the development of adenocarcinoma in humans. DRG-driven neurogenic inflammation in the spinal cord occurs early in the development of pancreatic ductal adenocarcinoma, promotes cancer associated pain and perineural invasion and is significantly reduced upon ablation of sensory neurons, slowing tumorigenesis and leading to the increase in overall survival [52, 63]. Direct effects of tumor on the sensory neurons and specific mechanisms by which DRG neurons may support pro-tumorigenic microenvironment remain largely undetermined. We have recently demonstrated that mouse B16 melanoma directly stimulates the growth of DRG neurons in vitro, altering their chemokine expression and leading to chemoattraction of MDSCs [64]. The presence of DRG neurons in the melanoma in vivo was associated with the enrichment of intra-tumoral granulocytic subset of MDSCs and the acceleration of tumor growth [64]. Therefore, the creation of immunosuppressive pro-tumorigenic microenvironment through the chemoattraction of MDSCs may be one way DRG neurons are utilized by cancer cells to support tumor progression.
Co-culture experiments of tumor cells and neurons demonstrate that human prostate cancer, pancreatic cancer and colon adenocarcinoma cells are able to induce neurite extensions by the neurons [65–68]. Reciprocal effect of neurons on cancer cells increases their migratory potential in vitro [69–71]. Based on the in vitro data, it is, therefore, reasonable to expect an enhanced density of nerve fibers within the tumor. However, while some studies do provide evidence of neurogenesis within the tumor [34, 66, 68, 72–74], others report normal or even reduced neuronal density within the tumor foci, and the presence of nerve fibers exclusively around the vasculature or in the peripheral stroma of the tumor [75–78]. The challenge in quantifying neuronal fibers by immunohistochemistry and the difficulty of differentiating between autonomic and sensory fibers in tissue may contribute to the discrepancy of reported tumor innervation. However, it is now clear that peripheral neurons, both afferent and efferent fibers, contribute to tumor progression through the modulation of the tumor microenvironment.
Neuroglia
Similar to the neuronal fibers, the glia of the CNS and the PNS are the source and target of chemokines, cytokines, neuropeptides and neurotrophic factors and participate in signaling with neurons, immune cells and other stromal components [53]. Glial cells such as astrocytes and Schwann cells express TLRs and Nod-like receptors which enables them to respond to neuronal injury, tissue damage or infection [79–81]. Neuroglia of the brain has been implicated in the pathogenesis of neurodegenerative diseases, traumatic brain injury and epilepsy [82–84], while peripheral glia has been extensively studied in the context of demyelinating diseases and peripheral neuropathy [85, 86]. Significantly, less is known about the role of glia in the tumor microenvironment.
The glia of the CNS has been largely investigated in the context of brain metastasis of breast or lung carcinoma. It is now well established that astrocytes are able to form gap junctions with the metastatic cancer cells, leading to the exchange of signaling molecules and the release of pro-inflammatory cytokines such as IFN-α and TNF-α [87]. The inflammatory microenvironment created by the astrocytes in this way further supports tumor growth and chemoresistance by activating NF-κB and STAT1 pathways and upregulating survival genes in breast and lung carcinoma cells [87–89]. The glia of the PNS has not been as rigorously studied. Specifically, how peripheral glia may contribute to tumorigenesis or tumor progression remains poorly understood.
Schwann cells
Schwann cells, the body’s most widely distributed neural crest-derived cells, form the major component of the PNS glia, functioning in myelination, axonal maintenance and repair [90–92]. As is the case with afferent neurons, Schwann cells also participate in neuropathic pain and may promote cancer-related analgesia [93–95]. Perhaps the best example of the direct interaction between Schwann cells and the tumor is illustrated in neuroblastoma—malignancy which originates from primitive sympathetic nervous system, where the presence of Schwann cell-rich stroma correlates with differentiated tumor cells and a more favorable prognosis [96, 97]. Low vascularity in Schwann cell-abundant stroma is another favorable prognostic factor in neuroblastoma, and part of the explanation may be attributed to an observation that Schwann cells can inhibit angiogenesis in vitro [98]. Another study described direct cross talk between neuroblastoma cells and Schwann cells via NRG1-mediated stimulation of Schwann cells by NTRK1-expressing tumor, leading to the expression of NGF by the activated Schwann cells, which in turn promoted the maturation of neuroblastoma [99]. The expression of neurotrophic factors by Schwann cells such as NRG1 and NGF and their tyrosine-kinase receptors of ErbB, p75 and Trk families is essential for Schwann cell development, proliferation, migration, as well as myelination and neuronal regeneration [90–92, 100]. Tumor types which share neural crest origin with Schwann cells, such as neuroblastoma and melanoma, also strongly rely on these signaling pathways. It is, therefore, reasonable to expect bidirectional communication between Schwann cells and tumor cells through neurotrophic and, likely, other factors. However, it is yet unclear whether these signaling pathways promote or inhibit tumor progression. For example, elevated expression of ErbB2 and ErbB3 carries an unfavorable prognosis in melanoma, breast and lung carcinoma, while the opposite appears to be true for neuroblastoma [101, 102]. Interestingly, recent study demonstrated a significant reduction of sympathetic nerve fibers, Schwann cells and nestin+ mesenchymal stem cells in the bone marrow of patients with myeloproliferative neoplasms and mouse model of the disease [103]. Nestin+ mesenchymal stem cells also originate from the neural crest, and together with their sympathetic innervation and supporting Schwann cells form hematopoietic stem cell niche, abrogation of which leads to myeloproliferative neoplasms [103, 104].
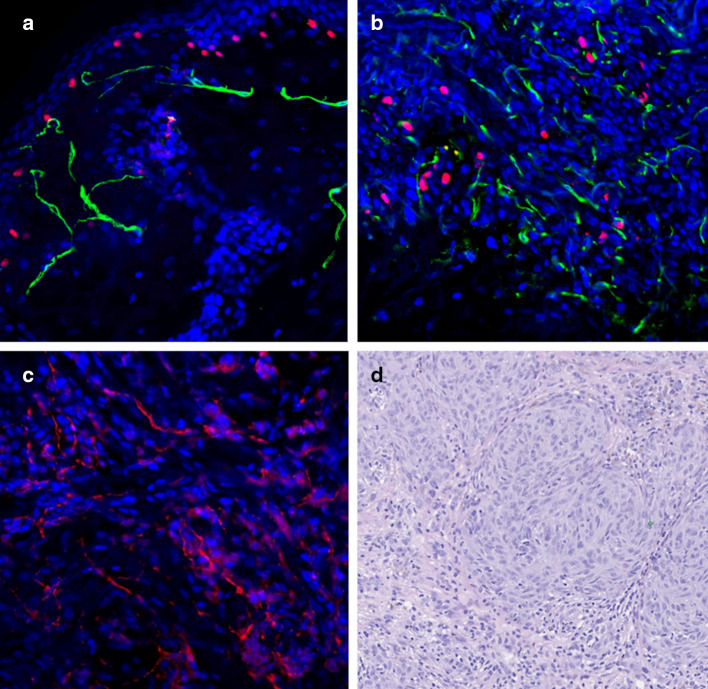
The presence of Schwann cells in the tumor stroma is a subject of some debate. Data supporting the migration of Schwann cells to the tumor parenchyma are contradicted by several examples of possible dedifferentiation of tumor cells to glial phenotype [105–109]. However, numerous examples in vitro and in vivo demonstrate mutual affinity of cancer cells and Schwann cells, supporting the hypothesis that Schwann cell presence in tumor microenvironment results from their migration. Schwann cells were found to migrate to the pancreatic intraepithelial neoplasm and intestinal adenoma in humans and mice before the onset of perineural invasion and advanced malignancy [110]. The strongest evidence for tumor cell-glial tropism comes from the models of perineural invasion [111]. Schwann cells may be able to promote perineural invasion of neurotropic tumor by direct contact with cancer cells in an integrin, NCAM-1, or MAG dependent manner [112–114]. We observe Schwann cells and neuronal fibers within the stroma of the invasive human melanoma (Fig. 1).
Fig. 1.
Co-localization of neuronal fibers, Schwann cells and malignant melanoma cells in human skin. Schwann cells and neuronal fibers in normal human skin and human melanoma were determined by immunohistochemistry. Representative fluorescent images of normal human skin (a) and human primary melanoma (b, c) are shown. H&E staining of human primary melanoma is also shown (d). Staining: red MITF, for melanocytes; green p75NGFR, for Schwann cells (a, b). Red PGP9.5, for neuronal fibers (c). Magnification: ×20
The abundance of Schwann cells in most organs and their virtually assured presence adjacent to early malignancy and within tumor microenvironment suggests that they may play a role in tumor progression or suppression. The question of how Schwann cells modulate tumor microenvironment remains largely unanswered. Schwann cells may interact with neuronal fibers and cancer cells directly, as well as with other stromal cells and immune cells. Furthermore, they are responsible for the remarkable robust regeneration response of the PNS after nerve injury [115, 116]. Axonal injury is followed by the loss of the neural fiber distal to the injured site (termed Wallerian degeneration), and the elimination of the contact between the axon and the supporting Schwann cells [117]. Those Schwann cells without contact with the axon undergo a series of signaling cascades leading to their dedifferentiation, proliferation and repair of the damaged nerve [116]. Within hours of nerve injury, supporting Schwann cells undergo an increase in the activity of multiple pathways, including Notch, JNK/c-Jun and ERK/MAPK [118, 119], and an increase in the expression of neurotrophic factors and their receptors, such as BDNF, GDNF, p75NTR and NRG1, as well as elevated secretion of cytokines and chemokines [116, 120]. Neuronal injury is followed by a local inflammatory response, and chemoattraction of macrophages in particular by the Schwann cells to the injured site aids in the clearance of myelin debris [117, 121, 122]. Interestingly, one study demonstrated that the process of Schwann cell dedifferentiation and immune cell recruitment in vivo may be triggered by the activation of only RAF/MEK/ERK pathway within the Schwann cells, even in the absence of nerve damage [123]. While the dedifferentiation of Schwann cells is critical to nerve injury response, these results suggest a possibility that activation of similar process may occur in other pathological states such as tumor invasion, and lead to Schwann cell modulation of the tumor microenvironment. Dysregulated tissue regeneration and chronic inflammation are the hallmarks of cancer [1, 124] in which Schwann cells could play a central role.
Schwann cells may be a link between cancer cells and a tumor-favorable immune response of the microenvironment. In vitro data suggest that Schwann cells may induce M2-phenotype in macrophages, which supports efficient repair of the peripheral nerves [125, 126]. The same phenotype of tumor-associated macrophages is correlated with worse clinical prognosis in malignancies [127]. Whether through a direct tumor activation or tumor-induced axonal and tissue injury, Schwann cells likely modulate the immune system and the tumor microenvironment.
Conclusions
Tumor microenvironment has attracted much attention, and numerous examples of non-malignant cell contribution to tumor progression are emerging. Nervous system may directly or indirectly influence the cancer cells and the tumor microenvironment. The best examples of such modulation are from the work on the sympathetic nervous system. Sensory fibers and neuroglia are less well studied, but the emerging evidence illustrates that their function in the tumor microenvironment is important. Schwann cells, in particular, add an exciting new dimensionality to the tumor–stroma interaction research. Schwann cells are extraordinarily plastic cells with rich profusion in most organs and a multitude of functions, and additional studies are warranted to further uncover their role in creating specific tumor microenvironment and supporting tumor progression.
Acknowledgements
The research is supported by P50CA121973 NIH Career Development Award to Y.L. Bunimovich.
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- CCR1
C-C chemokine receptor type 1
- CNS
Central nervous system
- CXCR4
C-X-C chemokine receptor type 4
- DRG
Dorsal root ganglia
- ERK
Extracellular signal-regulated kinase
- GDNF
Glial cell-derived neurotrophic factor
- HER2
Human epidermal growth factor receptor 2
- IFN-α
Interferon alpha
- IL
Interleukin
- IP-10
Interferon gamma-induced protein 10
- JNK c-Jun
N-terminal kinase
- MAG
Myelin-associated glycoprotein
- MAPK
Mitogen-activated protein kinase
- MCP-1
Monocyte chemoattractant protein 1
- MDSC
Myeloid-derived suppressor cell
- NCAM
Neural cell adhesion molecule
- NF-κB
Nuclear factor of kappa light polypeptide gene enhancer in B-cells
- NGF
Nerve growth factor
- NRG1
Neuregulin 1
- NTRK1
Neurotrophic receptor tyrosine kinase type 1
- P75NTR
p75 neurotrophin receptor
- PNS
Peripheral nervous system
- SNS
Sympathetic nervous system
- STAT1
Signal transducer and activator of transcription 1
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor alpha
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the conference Regulatory Myeloid Suppressor Cells: From Basic Discovery to Therapeutic Application which was hosted by the Wistar Institute in Philadelphia, PA, USA, 16th–19th June, 2016. It is part of a Cancer Immunology, Immunotherapy series of Focussed Research Reviews.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Gidron Y, Perry H, Glennie M. Does the vagus nerve inform the brain about preclinical tumours and modulate them? Lancet Oncol. 2005;6(4):245–248. doi: 10.1016/S1470-2045(05)70096-6. [DOI] [PubMed] [Google Scholar]
- 4.Green McDonald P, O’Connell M, Lutgendorf SK. Psychoneuroimmunology and cancer: a decade of discovery, paradigm shifts, and methodological innovations. Brain Behav Immun. 2013;30(Suppl):S1–S9. doi: 10.1016/j.bbi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tashiro M, Itoh M, Kubota K, Kumano H, Masud MM, Moser E, et al. Relationship between trait anxiety, brain activity and natural killer cell activity in cancer patients: a preliminary PET study. Psychooncology. 2001;10(6):541–546. doi: 10.1002/pon.548. [DOI] [PubMed] [Google Scholar]
- 6.Tashiro M, Kubota K, Itoh M, Nakagawa Y, Kamada M, Takahashi Y, et al. Regional cerebral glucose metabolism of patients with malignant diseases in different clinical phases. Med Sci Monit. 2001;7(2):226–232. [PubMed] [Google Scholar]
- 7.Golan H, Kennedy JA, Frenkel A, Parmet Y, Feintuch A, Levi O, et al. Brain mapping of patients with lung cancer and controls: inquiry into tumor-to-brain communication. J Nucl Med. 2009;50(7):1072–1075. doi: 10.2967/jnumed.108.061085. [DOI] [PubMed] [Google Scholar]
- 8.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry. 2003;54(3):269–282. doi: 10.1016/S0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 10.Palesh O, Butler LD, Koopman C, Giese-Davis J, Carlson R, Spiegel D. Stress history and breast cancer recurrence. J Psychosom Res. 2007;63(3):233–239. doi: 10.1016/j.jpsychores.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115(22):5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 12.Andersen BL, Farrar WB, Golden-Kreutz D, Emery CF, Glaser R, Crespin T, et al. Distress reduction from a psychological intervention contributes to improved health for cancer patients. Brain Behav Immun. 2007;21(7):953–961. doi: 10.1016/j.bbi.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyne JC, Stefanek M, Palmer SC. Psychotherapy and survival in cancer: the conflict between hope and evidence. Psychol Bull. 2007;133(3):367–394. doi: 10.1037/0033-2909.133.3.367. [DOI] [PubMed] [Google Scholar]
- 14.Stefanek ME, Palmer SC, Thombs BD, Coyne JC. Finding what is not there: unwarranted claims of an effect of psychosocial intervention on recurrence and survival. Cancer. 2009;115(24):5612–5616. doi: 10.1002/cncr.24671. [DOI] [PubMed] [Google Scholar]
- 15.Andersen BL, Yang HC, Farrar WB, Golden-Kreutz DM, Emery CF, Thornton LM, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113(12):3450–3458. doi: 10.1002/cncr.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kissane DW, Grabsch B, Clarke DM, Smith GC, Love AW, Bloch S, et al. Supportive-expressive group therapy for women with metastatic breast cancer: survival and psychosocial outcome from a randomized controlled trial. Psychooncology. 2007;16(4):277–286. doi: 10.1002/pon.1185. [DOI] [PubMed] [Google Scholar]
- 17.Lutgendorf SK, Sood AK, Antoni MH. Host factors and cancer progression: biobehavioral signaling pathways and interventions. J Clin Oncol. 2010;28(26):4094–4099. doi: 10.1200/JCO.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 19.Ebner K, Rupniak NM, Saria A, Singewald N. Substance P in the medial amygdala: emotional stress-sensitive release and modulation of anxiety-related behavior in rats. Proc Natl Acad Sci USA. 2004;101(12):4280–4285. doi: 10.1073/pnas.0400794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav Immun. 2003;17(5):321–328. doi: 10.1016/S0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 21.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92(12):994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 22.Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013;30(Suppl):S163–S170. doi: 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Schlossmacher G, Stevens A, White A. Glucocorticoid receptor-mediated apoptosis: mechanisms of resistance in cancer cells. J Endocrinol. 2011;211(1):17–25. doi: 10.1530/JOE-11-0135. [DOI] [PubMed] [Google Scholar]
- 24.Abduljabbar R, Negm OH, Lai CF, Jerjees DA, Al-Kaabi M, Hamed MR, et al. Clinical and biological significance of glucocorticoid receptor (GR) expression in breast cancer. Breast Cancer Res Treat. 2015;150(2):335–346. doi: 10.1007/s10549-015-3335-1. [DOI] [PubMed] [Google Scholar]
- 25.Antonova L, Mueller CR. Hydrocortisone down-regulates the tumor suppressor gene BRCA1 in mammary cells: a possible molecular link between stress and breast cancer. Genes Chromosomes Cancer. 2008;47(4):341–352. doi: 10.1002/gcc.20538. [DOI] [PubMed] [Google Scholar]
- 26.Sephton SE, Dhabhar FS, Keuroghlian AS, Giese-Davis J, McEwen BS, Ionan AC, et al. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. 2009;23(8):1148–1155. doi: 10.1016/j.bbi.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15(9):563–572. doi: 10.1038/nrc3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 29.Lutgendorf SK, DeGeest K, Sung CY, Arevalo JM, Penedo F, Lucci J, 3rd, et al. Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain Behav Immun. 2009;23(2):176–183. doi: 10.1016/j.bbi.2008.04.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA. 2013;110(41):16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanoun M, Maryanovich M, Arnal-Estape A, Frenette PS. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron. 2015;86(2):360–373. doi: 10.1016/j.neuron.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuller HM. Neurotransmission and cancer: implications for prevention and therapy. Anticancer Drugs. 2008;19(7):655–671. doi: 10.1097/CAD.0b013e3283025b58. [DOI] [PubMed] [Google Scholar]
- 33.Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18(5):1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341(6142):1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 35.Powe DG, Entschladen F. Targeted therapies: using beta-blockers to inhibit breast cancer progression. Nat Rev Clin Oncol. 2011;8(9):511–512. doi: 10.1038/nrclinonc.2011.123. [DOI] [PubMed] [Google Scholar]
- 36.Lamy S, Lachambre MP, Lord-Dufour S, Beliveau R. Propranolol suppresses angiogenesis in vitro: inhibition of proliferation, migration, and differentiation of endothelial cells. Vascul Pharmacol. 2010;53(5–6):200–208. doi: 10.1016/j.vph.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Lemeshow S, Sorensen HT, Phillips G, Yang EV, Antonsen S, Riis AH, et al. Beta-blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2273–2279. doi: 10.1158/1055-9965.EPI-11-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang D, Ma QY, Hu HT, Zhang M. Beta2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFkappaB and AP-1. Cancer Biol Ther. 2010;10(1):19–29. doi: 10.4161/cbt.10.1.11944. [DOI] [PubMed] [Google Scholar]
- 39.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20(7):754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang EV, Kim SJ, Donovan EL, Chen M, Gross AC, Webster Marketon JI, et al. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009;23(2):267–275. doi: 10.1016/j.bbi.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moretti S, Massi D, Farini V, Baroni G, Parri M, Innocenti S, et al. Beta-adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab Invest. 2013;93(3):279–290. doi: 10.1038/labinvest.2012.175. [DOI] [PubMed] [Google Scholar]
- 42.Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66(21):10357–10364. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 43.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson MB, Armaiz-Pena G, Takahashi R, Lin YG, Trevino J, Li Y, et al. Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J Biol Chem. 2007;282(41):29919–29926. doi: 10.1074/jbc.M611539200. [DOI] [PubMed] [Google Scholar]
- 45.Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70(18):7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armaiz-Pena GN, Gonzalez-Villasana V, Nagaraja AS, Rodriguez-Aguayo C, Sadaoui NC, Stone RL, et al. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget. 2015;6(6):4266–4273. doi: 10.18632/oncotarget.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armaiz-Pena GN, Allen JK, Cruz A, Stone RL, Nick AM, Lin YG, et al. Src activation by beta-adrenoreceptors is a key switch for tumour metastasis. Nat Commun. 2013;4:1403. doi: 10.1038/ncomms2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi M, Liu D, Duan H, Qian L, Wang L, Niu L, et al. The beta2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Res Treat. 2011;125(2):351–362. doi: 10.1007/s10549-010-0822-2. [DOI] [PubMed] [Google Scholar]
- 49.Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W, et al. A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature. 2011;477(7364):349–353. doi: 10.1038/nature10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolter JK, Wolter NE, Blanch A, Partridge T, Cheng L, Morgenstern DA, et al. Anti-tumor activity of the beta-adrenergic receptor antagonist propranolol in neuroblastoma. Oncotarget. 2014;5(1):161–172. doi: 10.18632/oncotarget.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peterson SC, Eberl M, Vagnozzi AN, Belkadi A, Veniaminova NA, Verhaegen ME, et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell. 2015;16(4):400–412. doi: 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saloman JL, Albers KM, Li D, Hartman DJ, Crawford HC, Muha EA, et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci USA. 2016;113(11):3078–3083. doi: 10.1073/pnas.1512603113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;194:417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhangoo SK, Ren D, Miller RJ, Chan DM, Ripsch MS, Weiss C, et al. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav Immun. 2007;21(5):581–591. doi: 10.1016/j.bbi.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leo M, Argalski S, Schäfers M, Hagenacker T. Modulation of voltage-gated sodium channels by activation of tumor necrosis factor receptor-1 and receptor-2 in small DRG neurons of rats. Mediators Inflamm. 2015;2015:124942. doi: 10.1155/2015/124942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013;2013:480739. doi: 10.1155/2013/480739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Exp Neurol. 2012;234(2):316–329. doi: 10.1016/j.expneurol.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Averbeck B, Izydorczyk I, Kress M. Inflammatory mediators release calcitonin gene-related peptide from dorsal root ganglion neurons of the rat. Neuroscience. 2000;98(1):135–140. doi: 10.1016/S0306-4522(00)00095-6. [DOI] [PubMed] [Google Scholar]
- 59.Rosa AC, Fantozzi R. The role of histamine in neurogenic inflammation. Br J Pharmacol. 2013;170(1):38–45. doi: 10.1111/bph.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khasabova IA, Stucky CL, Harding-Rose C, Eikmeier L, Beitz AJ, Coicou LG, et al. Chemical interactions between fibrosarcoma cancer cells and sensory neurons contribute to cancer pain. J Neurosci. 2007;27(38):10289–10298. doi: 10.1523/JNEUROSCI.2851-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Selvaraj D, Kuner R. Molecular players of tumor-nerve interactions. Pain. 2015;156(1):6–7. doi: 10.1016/j.pain.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 62.He S, Chen CH, Chernichenko N, He S, Bakst RL, Barajas F, et al. GFRalpha1 released by nerves enhances cancer cell perineural invasion through GDNF-RET signaling. Proc Natl Acad Sci USA. 2014;111(19):E2008–E2017. doi: 10.1073/pnas.1402944111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stopczynski RE, Normolle DP, Hartman DJ, Ying H, DeBerry JJ, Bielefeldt K, et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74(6):1718–1727. doi: 10.1158/0008-5472.CAN-13-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keskinov AA, Tapias V, Watkins SC, Ma Y, Shurin MR, Shurin GV. Impact of the sensory neurons on melanoma growth in vivo. PLoS ONE. 2016;11(5):e0156095. doi: 10.1371/journal.pone.0156095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ayala GE, Wheeler TM, Shine HD, Schmelz M, Frolov A, Chakraborty S, et al. In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate. 2001;49(3):213–223. doi: 10.1002/pros.1137. [DOI] [PubMed] [Google Scholar]
- 66.Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14(23):7593–7603. doi: 10.1158/1078-0432.CCR-08-1164. [DOI] [PubMed] [Google Scholar]
- 67.Li JH, Ma QY, Shen SG, Hu HT. Stimulation of dorsal root ganglion neurons activity by pancreatic cancer cell lines. Cell Biol Int. 2008;32(12):1530–1535. doi: 10.1016/j.cellbi.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 68.Albo D, Akay CL, Marshall CL, Wilks JA, Verstovsek G, Liu H, et al. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer. 2011;117(21):4834–4845. doi: 10.1002/cncr.26117. [DOI] [PubMed] [Google Scholar]
- 69.Ceyhan GO, Demir IE, Altintas B, Rauch U, Thiel G, Muller MW, et al. Neural invasion in pancreatic cancer: a mutual tropism between neurons and cancer cells. Biochem Biophys Res Commun. 2008;374(3):442–447. doi: 10.1016/j.bbrc.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 70.Dai H, Li R, Wheeler T, Ozen M, Ittmann M, Anderson M, et al. Enhanced survival in perineural invasion of pancreatic cancer: an in vitro approach. Hum Pathol. 2007;38(2):299–307. doi: 10.1016/j.humpath.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 71.Gil Z, Cavel O, Kelly K, Brader P, Rein A, Gao SP, et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010;102(2):107–118. doi: 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seifert P, Benedic M, Effert P. Nerve fibers in tumors of the human urinary bladder. Virchows Arch. 2002;440(3):291–297. doi: 10.1007/s004280100496. [DOI] [PubMed] [Google Scholar]
- 73.Seifert P, Spitznas M. Axons in human choroidal melanoma suggest the participation of nerves in the control of these tumors. Am J Ophthalmol. 2002;133(5):711–713. doi: 10.1016/S0002-9394(02)01329-6. [DOI] [PubMed] [Google Scholar]
- 74.Lu SH, Zhou Y, Que HP, Liu SJ. Peptidergic innervation of human esophageal and cardiac carcinoma. World J Gastroenterol. 2003;9(3):399–403. doi: 10.3748/wjg.v9.i3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitchell BS, Schumacher U, Stauber VV, Kaiserling E. Are breast tumours innervated? Immunohistological investigations using antibodies against the neuronal marker protein gene product 9.5 (PGP 9.5) in benign and malignant breast lesions. Eur J Cancer. 1994;30A(8):1100–1103. doi: 10.1016/0959-8049(94)90465-0. [DOI] [PubMed] [Google Scholar]
- 76.Terada T, Matsunaga Y. S-100-positive nerve fibers in hepatocellular carcinoma and intrahepatic cholangiocarcinoma: an immunohistochemical study. Pathol Int. 2001;51(2):89–93. doi: 10.1046/j.1440-1827.2001.01172.x. [DOI] [PubMed] [Google Scholar]
- 77.Zhou M, Patel A, Rubin MA. Prevalence and location of peripheral nerve found on prostate needle biopsy. Am J Clin Pathol. 2001;115(1):39–43. doi: 10.1309/2APJ-YKBD-97EH-67GW. [DOI] [PubMed] [Google Scholar]
- 78.Tomita T. Localization of nerve fibers in colonic polyps, adenomas, and adenocarcinomas by immunocytochemical staining for PGP 9.5. Dig Dis Sci. 2012;57(2):364–370. doi: 10.1007/s10620-011-1876-7. [DOI] [PubMed] [Google Scholar]
- 79.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA. 2005;102(16):5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, et al. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007;282(20):14975–14983. doi: 10.1074/jbc.M607277200. [DOI] [PubMed] [Google Scholar]
- 81.Lee H, Lee S, Cho IH, Lee SJ. Toll-like receptors: sensor molecules for detecting damage to the nervous system. Curr Protein Pept Sci. 2013;14(1):33–42. doi: 10.2174/1389203711314010006. [DOI] [PubMed] [Google Scholar]
- 82.Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353(6301):777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- 83.Chiu CC, Liao YE, Yang LY, Wang JY, Tweedie D, Karnati HK, et al. Neuroinflammation in animal models of traumatic brain injury. J Neurosci Methods. 2016;272:38–49. doi: 10.1016/j.jneumeth.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eyo UB, Murugan M, Wu LJ. Microglia-neuron communication in epilepsy. Glia. 2016 doi: 10.1002/glia.23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakano Y, Kanda T. Pathology of the peripheral nervous system in Guillain–Barre Syndrome. Brain Nerve. 2015;67(11):1329–1339. doi: 10.11477/mf.1416200303. [DOI] [PubMed] [Google Scholar]
- 86.Martini R, Willison H. Neuroinflammation in the peripheral nerve: cause, modulator, or bystander in peripheral neuropathies? Glia. 2016;64(4):475–486. doi: 10.1002/glia.22899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, et al. Carcinoma–astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533(7604):493–498. doi: 10.1038/nature18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim SJ, Kim JS, Park ES, Lee JS, Lin Q, Langley RR, et al. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia. 2011;13(3):286–298. doi: 10.1593/neo.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fidler IJ. The biology of brain metastasis: challenges for therapy. Cancer J. 2015;21(4):284–293. doi: 10.1097/PPO.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 90.Taveggia C. Schwann cells-axon interaction in myelination. Curr Opin Neurobiol. 2016;39:24–29. doi: 10.1016/j.conb.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 91.Jessen KR, Mirsky R, Lloyd AC. Schwann cells: development and role in nerve repair. Cold Spring Harb Perspect Biol. 2015;7(7):a020487. doi: 10.1101/cshperspect.a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Woodhoo A, Sommer L. Development of the Schwann cell lineage: from the neural crest to the myelinated nerve. Glia. 2008;56(14):1481–1490. doi: 10.1002/glia.20723. [DOI] [PubMed] [Google Scholar]
- 93.Mika J, Zychowska M, Popiolek-Barczyk K, Rojewska E, Przewlocka B. Importance of glial activation in neuropathic pain. Eur J Pharmacol. 2013;716(1–3):106–119. doi: 10.1016/j.ejphar.2013.01.072. [DOI] [PubMed] [Google Scholar]
- 94.Demir IE, Tieftrunk E, Schorn S, Saricaoglu OC, Pfitzinger PL, Teller S, et al. Activated Schwann cells in pancreatic cancer are linked to analgesia via suppression of spinal astroglia and microglia. Gut. 2016;65(6):1001–1014. doi: 10.1136/gutjnl-2015-309784. [DOI] [PubMed] [Google Scholar]
- 95.Campana WM. Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav Immun. 2007;21(5):522–527. doi: 10.1016/j.bbi.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brodeur GM. Schwann cells as antineuroblastoma agents. N Engl J Med. 1996;334(23):1537–1539. doi: 10.1056/NEJM199606063342311. [DOI] [PubMed] [Google Scholar]
- 97.Liu S, Tian Y, Chlenski A, Yang Q, Zage P, Salwen HR, et al. Cross-talk between Schwann cells and neuroblasts influences the biology of neuroblastoma xenografts. Am J Pathol. 2005;166(3):891–900. doi: 10.1016/S0002-9440(10)62309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang D, Rutkowski JL, Brodeur GM, Chou PM, Kwiatkowski JL, Babbo A, et al. Schwann cell-conditioned medium inhibits angiogenesis. Cancer Res. 2000;60(21):5966–5971. [PubMed] [Google Scholar]
- 99.Pajtler KW, Mahlow E, Odersky A, Lindner S, Stephan H, Bendix I, et al. Neuroblastoma in dialog with its stroma: NTRK1 is a regulator of cellular cross-talk with Schwann cells. Oncotarget. 2014;5(22):11180–11192. doi: 10.18632/oncotarget.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Newbern J, Birchmeier C. Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin Cell Dev Biol. 2010;21(9):922–928. doi: 10.1016/j.semcdb.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wilzen A, Krona C, Sveinbjornsson B, Kristiansson E, Dalevi D, Ora I, et al. ERBB3 is a marker of a ganglioneuroblastoma/ganglioneuroma-like expression profile in neuroblastic tumours. Mol Cancer. 2013;12(1):70. doi: 10.1186/1476-4598-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reschke M, Mihic-Probst D, van der Horst EH, Knyazev P, Wild PJ, Hutterer M, et al. HER3 is a determinant for poor prognosis in melanoma. Clin Cancer Res. 2008;14(16):5188–5197. doi: 10.1158/1078-0432.CCR-08-0186. [DOI] [PubMed] [Google Scholar]
- 103.Arranz L, Sanchez-Aguilera A, Martin-Perez D, Isern J, Langa X, Tzankov A, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512(7512):78–81. doi: 10.1038/nature13383. [DOI] [PubMed] [Google Scholar]
- 104.Isern J, Garcia-Garcia A, Martin AM, Arranz L, Martin-Perez D, Torroja C, et al. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife. 2014;3:e03696. doi: 10.7554/eLife.03696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mora J, Cheung NK, Juan G, Illei P, Cheung I, Akram M, et al. Neuroblastic and Schwannian stromal cells of neuroblastoma are derived from a tumoral progenitor cell. Cancer Res. 2001;61(18):6892–6898. [PubMed] [Google Scholar]
- 106.Bourdeaut F, Ribeiro A, Paris R, Pierron G, Couturier J, Peuchmaur M, et al. In neuroblastic tumours, Schwann cells do not harbour the genetic alterations of neuroblasts but may nevertheless share the same clonal origin. Oncogene. 2008;27(21):3066–3071. doi: 10.1038/sj.onc.1210965. [DOI] [PubMed] [Google Scholar]
- 107.Iyengar B, Singh AV. Patterns of neural differentiation in melanomas. J Biomed Sci. 2010;17:87. doi: 10.1186/1423-0127-17-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52(2):119–129. doi: 10.1111/j.1365-2559.2007.02823.x. [DOI] [PubMed] [Google Scholar]
- 109.Van Raamsdonk CD, Deo M. Links between Schwann cells and melanocytes in development and disease. Pigment Cell Melanoma Res. 2013;26(5):634–645. doi: 10.1111/pcmr.12134. [DOI] [PubMed] [Google Scholar]
- 110.Demir IE, Boldis A, Pfitzinger PL, Teller S, Brunner E, Klose N, et al. Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. J Natl Cancer Inst. 2014 doi: 10.1093/jnci/dju184. [DOI] [PubMed] [Google Scholar]
- 111.Liu H, Li X, Xu Q, Lv S, Li J, Ma Q. Role of glial cell line-derived neurotrophic factor in perineural invasion of pancreatic cancer. Biochim Biophys Acta. 1826;1:112–120. doi: 10.1016/j.bbcan.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 112.Sroka IC, Chopra H, Das L, Gard JM, Nagle RB, Cress AE. Schwann cells increase prostate and pancreatic tumor cell invasion using laminin binding A6 integrin. J Cell Biochem. 2016;117(2):491–499. doi: 10.1002/jcb.25300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deborde S, Omelchenko T, Lyubchik A, Zhou Y, He S, McNamara WF, et al. Schwann cells induce cancer cell dispersion and invasion. J Clin Invest. 2016;126(4):1538–1554. doi: 10.1172/JCI82658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Swanson BJ, McDermott KM, Singh PK, Eggers JP, Crocker PR, Hollingsworth MA. MUC1 is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Res. 2007;67(21):10222–10229. doi: 10.1158/0008-5472.CAN-06-2483. [DOI] [PubMed] [Google Scholar]
- 115.Kidd GJ, Ohno N, Trapp BD. Biology of Schwann cells. Handb Clin Neurol. 2013;115:55–79. doi: 10.1016/B978-0-444-52902-2.00005-9. [DOI] [PubMed] [Google Scholar]
- 116.Glenn TD, Talbot WS. Signals regulating myelination in peripheral nerves and the Schwann cell response to injury. Curr Opin Neurobiol. 2013;23(6):1041–1048. doi: 10.1016/j.conb.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sheu JY, Kulhanek DJ, Eckenstein FP. Differential patterns of ERK and STAT3 phosphorylation after sciatic nerve transection in the rat. Exp Neurol. 2000;166(2):392–402. doi: 10.1006/exnr.2000.7508. [DOI] [PubMed] [Google Scholar]
- 119.Woodhoo A, Alonso MB, Droggiti A, Turmaine M, D’Antonio M, Parkinson DB, et al. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat Neurosci. 2009;12(7):839–847. doi: 10.1038/nn.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stassart RM, Fledrich R, Velanac V, Brinkmann BG, Schwab MH, Meijer D, et al. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat Neurosci. 2013;16(1):48–54. doi: 10.1038/nn.3281. [DOI] [PubMed] [Google Scholar]
- 121.Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci. 2002;22(15):6696–6703. doi: 10.1523/JNEUROSCI.22-15-06696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Benowitz LI, Popovich PG. Inflammation and axon regeneration. Curr Opin Neurol. 2011;24(6):577–583. doi: 10.1097/WCO.0b013e32834c208d. [DOI] [PubMed] [Google Scholar]
- 123.Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, et al. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73(4):729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 124.Pesic M, Greten FR. Inflammation and cancer: tissue regeneration gone awry. Curr Opin Cell Biol. 2016;43:55–61. doi: 10.1016/j.ceb.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 125.Stratton JA, Shah PT, Kumar R, Stykel MG, Shapira Y, Grochmal J, et al. The immunomodulatory properties of adult skin-derived precursor Schwann cells: implications for peripheral nerve injury therapy. Eur J Neurosci. 2016;43(3):365–375. doi: 10.1111/ejn.13006. [DOI] [PubMed] [Google Scholar]
- 126.Stratton JA, Shah PT. Macrophage polarization in nerve injury: do Schwann cells play a role? Neural Regen Res. 2016;11(1):53–57. doi: 10.4103/1673-5374.175042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev. 2016;99(Pt B):180–185. doi: 10.1016/j.addr.2015.11.009. [DOI] [PubMed] [Google Scholar]