Abstract
Mounting evidence supports a mechanistic link between inflammation and cancer, especially colon cancer. ALOX15 (15-lipoxygenase-1) plays an important role in the formation of key lipid mediators (e.g., lipoxins and resolvins) to terminate inflammation. ALOX15 expression is downregulated in colorectal cancer (CRC). Intestinally-targeted transgenic expression of ALOX15 in mice inhibited dextran sodium sulfate-induced colitis from promoting azoxymethane- induced colorectal tumorigenesis, demonstrating that ALOX15 can suppress inflammation-driven promotion of carcinogen-induced colorectal tumorigenesis and therefore ALOX15 downregulation during tumorigenesis is likely to enhance the link between colitis and colorectal tumorigenesis. ALOX15 suppressed the TNF-α, IL-1β/NF-κB, and IL-6/STAT3 signaling pathways, which play major roles in promotion of colorectal cancer by chronic inflammation. Defining ALOX15’s regulatory role in colitis-associated colorectal cancer could identify important molecular regulatory events that could be targeted to suppress promotion of tumorigenesis by chronic inflammation.
Keywords: ALOX15, Colon Cancer, Colitis-Associated Colorectal Cancer
Introduction
Evidence is mounting that a mechanistic link exists between inflammation and cancer [1], especially colonic cancer [2]. Colitis induced chemically in mice by dextran sodium sulfate strongly enhances colorectal carcinogenesis [3]. Similarly, mouse models of genetically-induced colitis, e.g., through IL-10 knock-out [4] or glutathione peroxidase-1 and peroxidase-2 isozyme knock-out [5], also show enhanced colorectal carcinogenesis [6]. In humans, inflammatory bowel diseases (ulcerative colitis and Crohn’s disease) markedly increase colorectal cancer risk [6, 7], and colon cancer accounts for an estimated 15% of deaths in patients with ulcerative colitis [8]. Although differences in molecular pathogenesis exist between colitis-associated colorectal cancer and the more common sporadic colorectal cancer [2], some chronic inflammatory mechanisms (e.g., cyclooxygenase-2 overexpression) contribute significantly to both [6]. Thus, studying the mechanisms by which chronic inflammation promotes colonic tumorigenesis could also provide insights into the pathogenesis of sporadic colorectal tumorigenesis.
The development and maintenance of chronic inflammation is strongly influenced by oxidative metabolism of polyunsaturated fatty acids (PUFAs) [9]. PUFA oxidative metabolism is enzymatically regulated in cells via several groups of enzymes, the best known of which are the cyclooxygenases (COXs), lipoxygenases (LOXs), and cytochrome p450s (CYP450s) [10]. The roles of cyclooxygenases and cytochrome p450 enzymes in inflammation and cancer have been studied extensively in the literature [10, 11]. The current review will focus on the role of LOXs, especially ALOX15 (human 15-lipoxygenase-1; mouse 12/15-lipoxygenase), in chronic inflammation and cancer.
LOXs metabolize PUFAs and thereby regulate inflammation and its resolution
LOXs are dioxygenase enzymes that incorporate oxygen into PUFAs (e.g., arachidonic acid (AA) or linoleic acid (LA)) to form biologically-active peroxide products (e.g., hydroperoxyeicosatetraenoic acids (HpETEs) or hydroperoxyoctadecadienoic acid HpODEs) [12, 13]. LOXs are named according to the specific location in the arachidonic acid carbon chain where the enzyme catalyzes lipid peroxidation (e.g., ALOX12 oxygenates arachidonic acid at the 12th carbon). Human LOX genes include ALOX5, ALOXE3, ALOX12, ALOX12B, ALOX15, and ALOX15B; mice share these 6 genes, and an additional skin-specific 12-LOX (Alox12e), which is a pseudogene in humans [12, 14].
While products of LOX-mediated AA metabolism (e.g., 5-HETE and leukotriene B4 (LTB4) from 5-LOX-mediated metabolism) contribute to the initiation of acute inflammation [15], other products of LOX-mediated metabolism of PUFAs (lipoxins (from AA), resolvins (from docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), protectins (DHA), and maresins (DHA)) are critical to the active process of inflammation resolution, failure of which allows for the development of chronic inflammation [9].
ALOX15 regulates inflammation through multiple pathways
Mammalian ALOX15 is an inducible and highly regulated enzyme in normal cells and evidence reveals it can counterregulate pro-inflammatory signaling via multiple mechanisms [16]. ALOX15 is most commonly known as the rate-limiting enzyme for production of 13-S-HODE from LA [17, 18]. 13-S-HODE is an activating ligand of peroxisome proliferator-activated receptor gamma (PPARγ) and suppressor of PPAR delta (PPARδ) [19–21]. PPARγ inhibits inflammation [22], while PPARδ promotes inflammation, especially colitis [23]. Studies with 12/15-LOX, the mouse homolog of human ALOX15, have suggested that 12/15-LOX plays both pro-inflammatory and anti-inflammatory roles due to its higher ratio of 12- to 15-lipoxygenase activity, and therefore higher levels of the pro-inflammatory mediator 12-S-HETE [24]. In humans, however, several lines of evidence suggest that ALOX15 plays an anti-inflammatory role. Overexpression of human ALOX15 inhibits polymorphonuclear-cell-mediated tissue destruction in rabbits [25] and glomerulonephritis in rats [26]. ALOX15 activates PPARγ through 13-S-HODE [20, 27]. PPARγ activation inhibits colitis [22] and colitis-associated colonic tumorigenesis [28]. Further evidence of an anti-inflammatory role of human ALOX15 comes from studies of its impact on interleukin-1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α). These molecules are major pro-inflammatory cytokines that contribute to the pathogenesis of human colitis; TNF-α-blocking agents are used to treat ulcerative colitis [29, 30]. Downregulation of ALOX15 expression in human colorectal cancer cells is associated with upregulation of IL-1β, and re-expression of ALOX15 in colon cancer cells suppresses IL-1β expression [31]. Furthermore, transgenic expression of human ALOX15 in mouse colonic epithelial cells inhibits TNF-α and nuclear factor kappa B (NF-κB) signaling [32].
While the role of 13-S-HODE in inhibiting inflammation is less established, resolvins and lipoxins, which are products of ALOX15-mediated metabolism of EPA or DHA and AA, respectively, have been demonstrated to play critical roles in resolution of inflammation [9]. Termination of the acute inflammatory phase has been shown to involve lipid mediator class switching of arachidonic acid metabolites from pro-inflammatory eicosanoids (e.g., prostaglandin E2 and leukotriene B4) to pro-resolving mediators such as lipoxins (e.g., lipoxin A4 and lipoxin B4) [9]. This shift in eicosanoid biosynthesis is dependent upon upregulation of ALOX15, which is critical to lipoxin biosynthesis [33, 34].
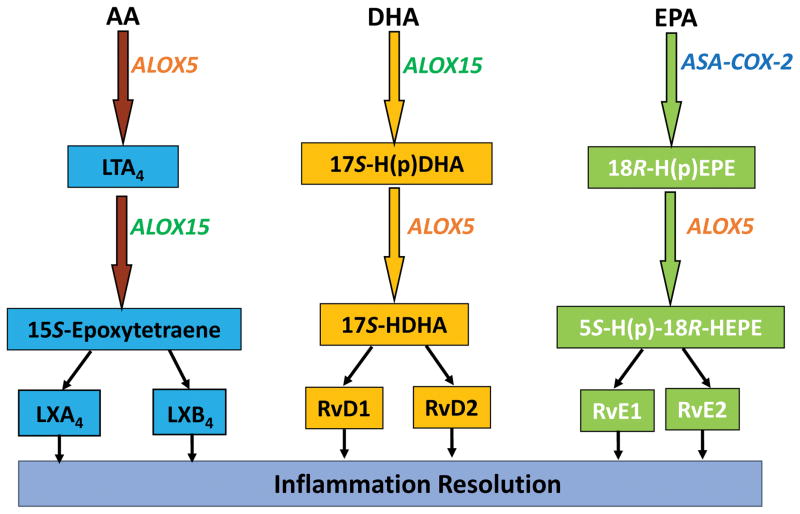
ALOX15 also contributes to the generation of resolvins, which are among the best-known pro-resolving mediators. The resolvins are oxidative metabolites of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA): the D-series resolvins (e.g., RvD1) are derived from DHA; the E-series resolvins (e.g., RvE1) are derived from EPA [35] (Figure 1). ALOX15 enzymatic function is critical to the generation of the RvD precursor 17-S-HpDHA from DHA [36, 37]. 15-LOX-like function of aspirin-acetylated COX-2 catalyzes generation of the RvE precursor 18-HEPE from EPA [37, 38]. Resolvins have demonstrated strong anti-inflammatory impacts (picomolar to nanomolar range) in various in vivo preclinical models of chronic inflammatory disease, including colitis [39, 40]. For example, RvE1, RvD1, and RvD2 inhibit chemically-induced colitis in mice [41, 42]. RvD1 markedly reduces IL-6, IL-1β, and TNF-α expression in various experimental models [36].
Figure 1. Enzymes involved in biosynthesis of lipoxins (LXs) and resolvins (Rvs) from long-chain PUFA.
Multiple PUFA can be metabolized by lipoxygenases, including ALOX15. Shown here are the known pathways involved in biosynthesis of several key classes of SPMs from arachidonic acid (AA), docoahexaenoic acid (DHA), and eicosapentaenoic acid (EPA). Note that the generation of bioactive mediators can involve multiple enzymatic steps and transcellular modes of biosynthesis have been described for SPM [35].
While ALOX15 is known to be expressed in the epithelial compartment, it is also present in other cells types, including leukocytes (e.g., neutrophils, macrophages) and vascular endothelial cells (reviewed in [43, 44]). Macrophages show a great deal of heterogeneity in terms of their biomarkers and actions within different tissues, dependent upon host status (healthy, injured, malignant, etc.) [45, 46], and the role of ALOX15 has been investigated in the context of macrophage phenotype [47–49]. The subsets of macrophages involved in resolution of acute inflammatory responses actively remove apoptotic cells and debris, and promote repair of damaged tissues [45, 50, 51]. Resolution-phase macrophages from resolving murine peritonitis were described as “M2-like”, expressing high IL-10, TGF-β, and arginase-1, low IL-12, and increased 12/15-LOX [50, 52]. Additional work using the peritonitis model uncovered distinctions between early and late resolution-phase macrophages [47]. Here, populations of F4/80+ macrophages from resolving exudates were distinguished in part on the basis of CD11b expression; CD11bhigh macrophages had low levels of M1 markers, moderate expression of pro-inflammatory cytokines and chemokines, and low 12/15-LOX, while CD11blow macrophages showed reduced pro-inflammatory cytokines/chemokines, low IL-10, and higher 12/15-LOX and TGFβ. In addition to differences in markers, CD11bhigh macrophages were efficient phagocytic cells, whereas CD11blow macrophages ceased phagocytosing apoptotic PMN, and were described as “satiated”. Satiated macrophages were also more likely to emigrate to draining lymph nodes, where they are involved in modulation or termination of adaptive immune responses [47][49]. Interestingly, satiated efferocytosis was promoted in the peritonitis model by addition of resolvins E1 and D1 (a 12/15-LOX metabolite). ALOX15 expression can be induced in macrophages through interactions with/engulfment of apoptotic cells; it is also inducible by IL-4 and IL-13 [52–54], and galectin-1 [55]. Interestingly, mouse ALOX15 (12/15-LOX) has been shown to control uptake of apoptotic cells by different macrophage subsets, helping to limit inappropriate immune responses [56]. Alterations in the IL-10 signaling pathway have also been implicated in development of chronic inflammatory states in the colon [57];reviewed in [58, 59]. IL-10 is considered an important anti-inflammatory mediator and macrophage-specific deficits in IL-10 signaling can lead to severe inflammation in the colon [58, 60, 61]. Evidence has shown that specialized pro-resolving mediators (e.g., RvD1) requiring ALOX15 for biosynthesis have been shown to increase IL-10 levels in models of acute inflammation [36, 62]. More specifically, both DHA and RvD1 have been shown to drive adipose tissue macrophages towards an M2-like phenotype [63]. Given the accumulating data on ALOX15 expression, SPM biosynthesis and/or responsiveness in macrophage subpopulations, more attention should be placed on how ALOX15/SPMs may influence IL-10 signaling in the intestine under normal and pathological states.
Under homeostatic conditions, gut macrophages have an anti-inflammatory or M2 polarization, playing a key role in maintaining a tolerogenic environment [64, 65]. In the setting of chronic inflammatory disease (UC, Crohn’s) or neoplastic progression, macrophage phenotype can be altered [65]. Although tumor-associated macrophages (TAMs) are considered to act in a protumorigenic manner, in part through proangiogenic and immunosuppressive mechanisms [66], there is controversy over whether macrophages in CRC represent a good prognostic indicator or not [65, 67, 68]. Many issues still surround TAMs, for example, the precise origin (e.g., tissue resident or monocyte-derived) of these cells at earlier stages of tumor development is unclear, and whether these cells can act to control early stages of cancer (preneoplastic lesions) remains to be determined [66]. In the context of CRC, macrophage populations may differ depending on whether the cancer arose in a chronically inflamed tissue or represents a sporadic lesion. To date, the ALOX15 status of macrophages (and other stromal cell types) associated with tumor development in colon has not been studied in depth, but given that M2-like or pro-resolving macrophages express ALOX15 and M2-like macrophages are key in regulating the intestinal microenvironment, there is support for the concept that ALOX15+ macrophages have regulatory functions limiting colitis and subsequent promotion of colorectal tumorigenesis. Mechanistic studies to clearly confirm this role are needed.
ALOX15 inhibits colorectal tumorigenesis
ALOX15 expression is lost early in colorectal tumorigenesis, starting at the premalignant adenoma phase [69–72]. In contrast, other LOXs do not appear to be significantly altered during colonic tumorigenesis [31, 73, 74]. Downregulation of ALOX15 expression has also been reported in various other human cancers, including lung [75], esophageal [76], breast [77], endometrial [78], urinary bladder [79] and pancreatic cancer [80]. Additionally, screening of 128 different human cancer cell lines representing 20 different human cancers, including all common human cancers, showed that ALOX15 expression was markedly repressed [75]. Loss of ALOX15 expression is transcriptionally mediated [81] and independent of substrate availability [31]. While some earlier studies suggested that ALOX15 might have a procarcinogenic role, several lines of evidence, including more recent evidence [32, 82] have demonstrated that ALOX15 has a tumor-suppressing role, especially in colorectal tumorigenesis [74, 83]. ALOX15 re-expression in human colorectal cancer cells via pharmaceutical agents [21, 84, 85] or plasmid or adenoviral vectors [20, 70, 73] inhibits the growth of those cells in vitro and in vivo [86]. Transgenic expression of human ALOX15 in mouse colonocytes (ALOX15-Gut mice) inhibits colorectal tumorigenesis [32]. ALOX15 expression in ALOX15-Gut mice inhibits NF-κB activation and azoxymethane-induced colorectal tumorigenesis [32] and colitis-associated colorectal tumorigenesis [82].
ALOX15 inhibits colitis-driven promotion of colorectal tumorigenesis
NF-κB and STAT3 cooperate to promote colitis-associated colorectal cancer [87]. We studied whether ALOX15 influenced STAT3 signaling in colitis-promoted colorectal tumorigenesis. We found that the acceleration of azoxymethane-induced colorectal tumorigenesis by dextran sodium sulfate-driven colitis was inhibited by ALOX15 transgenic expression in colonic epithelial cells [82]. Inhibition of tumor development/progression in this model was associated with suppression of both IL-6 expression and subsequent STAT3 phosphorylation and signaling, thereby limiting expression of protumorigenic STAT3-driven genes Notch3 and Muc1. Similarly, in human colon cancer cells, re-expression of ALOX15 downregulated IL-6/STAT3 signaling [82], thus demonstrating the translational relevance of the ALOX15 transgenic mouse model results to human colonic tumorigenesis.
ALOX15 exerts important modulatory effects on PPARγ and PPARδ, which are lipid nuclear receptors that function as master regulators of various important cellular events [e.g. metabolism [88], inflammation [89], and tumorigenesis[90]]. While PPARγ is considered to have an antitumorigenic role, the role of PPARδ in tumorigenesis was felt to be controversial [90]. Nevertheless, PPARδ can play an antagonistic role to PPARγ during tumorigenesis [20], and mounting data are confirming the strong protumorigenic role for PPARδ [23, 91, 92]. As mentioned earlier, ALOX15, via 13-S-HODE production, downregulates PPARδ [21]. PPARδ promotes colitis and IL-6 expression [23]. However, prior results regarding the role of PPARδ in intestinal tumorigenesis were contradictory: Ppard germline knock-out in APCmin mice increased intestinal tumorigenesis in one mouse model [93] but inhibited it in another [94]. In contrast, in the azoxymethane-induced intestinal carcinogenesis model, which better simulates human colonic tumorigenesis, intestinally-targeted Ppard genetic deletion profoundly inhibited colonic tumorigenesis [95]. Moreover, intestinally-targeted Ppard overexpression resulted in strong promotion of azoxymethane-induced tumorigenesis [91]. Cross-breeding of mice with intestinally-targeted Ppard overexpression with ALOX15 transgenic mice confirmed in vivo the ability of ALOX15-mediated signaling to suppress PPARδ and downstream signaling through IL-6/STAT3, thereby limiting the development of colitis-associated colon cancer [82]. ALOX15 suppression of PPARδ/IL-6/STAT3 signaling also strongly inhibited expression of MUC1 [82], which activates proinflammatory, protumorigenic pathways in colon cancer (e.g., NF-κB) [96] and promotes colitis-associated colon cancer [97].
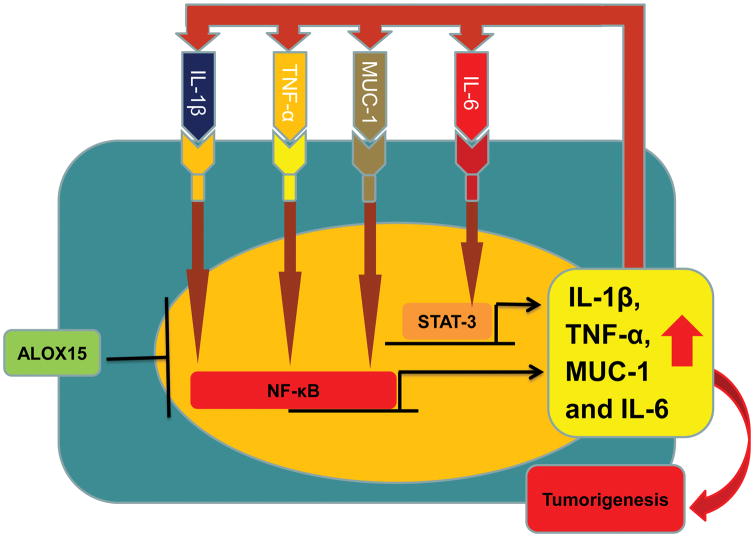
On the basis of these findings and our prior findings of ALOX15 repression of TNF-α and IL-1β as drivers of NF-κB signaling [32], we propose a theoretical model in which ALOX15 interrupts positive feedback cycles between proinflammatory factors and NF-κB and STAT3 to inhibit tumorigenesis (Figure 2). These findings support the concept that ALOX15 downregulation during tumorigenesis further augments colitis promotion of colonic tumorigenesis, thus strengthening the link between these two pathological processes.
Figure 2. Schematic representation of proposed theoretical model for ALOX15 inhibition of cytokine-driven NF-κB and STAT3 enhancement of IL-6, IL-1β, MUC1 and TNF-α transcription and tumorigenesis promotion.
As shown here and discussed in the text, ALOX15 impacts pro-tumorigenic signaling via multiple pathways including suppression of IL-6, IL-1β, TNF-α, STAT3 and NFκB signaling. STAT3 and NFκB are both key transcription factors associated with promotion of inflammation-driven tumorigenesis in the gut [87]. NFκB activity is enhanced by a number of cytokines, such IL-1β, TNF-α, as well as the glycoprotein MUC1. IL-6, an NFκB- responsive gene, can lead to upregulation of STAT3 signaling. In epithelial cancer cells as well as in the tumor microenvironment, dysregulation of these pathways leads to sustained inflammation through feed-forward mechanisms. While detailed mechanisms involved in ALOX15’s ability to act as a brake on colorectal tumorigenesis by suppressing these pathways have not been worked out, they likely involve pro-resolving ALOX15 metabolites (e.g., lipoxins, resolvins, etc.) signaling.
Future questions to be answered
The literature to date regarding the contribution of ALOX15 to colonic tumorigenesis has been focused on the role of ALOX15 in colonic epithelial cells. The likely reason for this focus is that ALOX15 loss has been observed in epithelial but not in stromal cells in cancer [69]. Given the demonstration that ALOX15 expression in leukocytes is critical in mediating the lipid mediator class switching to resolve acute inflammation, it is important to address the role of ALOX15 activity in populations of cells that make up the tumor microenvironment. It is currently unknown whether ALOX15 suppression in various leukocyte subclasses is involved in the tumor promotion by chronic inflammation or conversely, whether increasing ALOX15 expression or activity in these cells might help limit tumor development. Further studies to determine ALOX15’s expression and actions in classes of tumor-associated leukocytes are therefore warranted. As the biosynthesis of many specialized pro-resolving mediators (e.g. lipoxins, resolvins) from PUFA precursors requires multiple enzymatic steps, and can involve transcellular mechanisms of biosynthesis, it will be important to address potential relationships between cell populations in order to fully understand ALOX15’s roles in tumor biology. Additional studies are also needed to determine whether the regulatory role of ALOX15 in suppressing inflammation-driven tumorigenesis is specific to colon cancer or also applies to other cancers.
Conclusion
Emerging data show that ALOX15 is an important regulator of major signaling pathways (e.g., TNF-α, IL-1β/NF-κB, and IL-6/STAT3) that promote colitis-associated colon cancer. Further defining this role of ALOX15 could identify important molecular regulatory events that could be targeted to suppress colitis-associated colonic tumorigenesis in particular and possibly inflammation-driven promotion of tumorigenesis in general.
Supplementary Material
Highlights.
ALOX15 plays an important role in the formation of key lipid mediators (e.g., lipoxins and resolvins) to terminate inflammation.
ALOX15 expression is downregulated in colon cancer.
ALOX15 most likely plays an important regulatory role in suppressing signaling pathways (e.g., NF-κB and STAT3) that promote colitis-associated colonic tumorigenesis.
Acknowledgments
This work was partially supported by the National Cancer Institute through grants R01-CA 206539 and R01-CA 1095686 and by the Cancer Prevention Research Institute of Texas through grants RP140224 and RP150195 to I.S. The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant CA016672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118(6):671–4. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protocols. 2007;2(8):1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- 4.Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98(4):1010–20. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu FF, Esworthy RS, Chu PG, Longmate JA, Huycke MM, Wilczynski S, Doroshow JH. Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Res. 2004;64(3):962–8. doi: 10.1158/0008-5472.can-03-2272. [DOI] [PubMed] [Google Scholar]
- 6.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287(1):G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 7.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323(18):1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 8.Breynaert C, Vermeire S, Rutgeerts P, Van Assche G. Dysplasia and colorectal cancer in inflammatory bowel disease: a result of inflammation or an intrinsic risk? Acta Gastroenterologica Belgica. 2008;71(4):367–72. [PubMed] [Google Scholar]
- 9.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Zhu J, Lyu F, Panigrahy D, Ferrara KW, Hammock B, Zhang G. ω-3 Polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins & Other Lipid Mediators. 2014;113–115:13–20. doi: 10.1016/j.prostaglandins.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, DuBois RN. PROSTAGLANDINS AND CANCER. Gut. 2006;55(1):115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274(34):23679–82. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 13.Shureiqi I, Lippman SM. Lipoxygenase modulation to reverse carcinogenesis. Cancer Res. 2001;61(17):6307–12. [PubMed] [Google Scholar]
- 14.Muñoz-Garcia A, Thomas CP, Keeney DS, Zheng Y, Brash AR. The importance of the lipoxygenase-hepoxilin pathway in the mammalian epidermal barrier. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2014;1841(3):401–408. doi: 10.1016/j.bbalip.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funk CD. Prostaglandins and Leukotrienes: Advances in Eicosanoid Biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn H, Walther M, Kuban RJ. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002;68–69:263–90. doi: 10.1016/s0090-6980(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 17.Baer AN, Costello PB, Green FA. In vivo activation of an omega-6 oxygenase in human skin. Biochem Biophys Res Commun. 1991;180(1):98–104. doi: 10.1016/s0006-291x(05)81260-4. [DOI] [PubMed] [Google Scholar]
- 18.Brash AR, Boeglin WE, Chang MS. Discovery of a second 15S-lipoxygenase in humans. Proc Natl Acad Sci U S A. 1997;94(12):6148–52. doi: 10.1073/pnas.94.12.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takamitsu S, Kiyomu F, Kazuhiro Y, Hideo S, Tomonori S, Hitoshi O, Hiroki K. Peritoneal metastasis inhibition by linoleic acid with activation of PPARγ in human gastrointestinal cancer cells. Virchows Archiv. 2006;448(4):422–427. doi: 10.1007/s00428-005-0110-4. [DOI] [PubMed] [Google Scholar]
- 20.Zuo X, Wu Y, Morris JS, Stimmel JB, Leesnitzer LM, Fischer SM, Lippman SM, Shureiqi I. Oxidative metabolism of linoleic acid modulates PPAR-beta/delta suppression of PPAR-gamma activity. Oncogene. 2006;25(8):1225–41. doi: 10.1038/sj.onc.1209160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shureiqi I, Jiang W, Zuo X, Wu Y, Stimmel JB, Leesnitzer LM, Morris JS, Fan HZ, Fischer SM, Lippman SM. The 15-lipoxygenase-1 product 13-S-hydroxyoctadecadienoic acid down-regulates PPAR-delta to induce apoptosis in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100(17):9968–73. doi: 10.1073/pnas.1631086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su CG, Wen X, Bailey ST, Jiang W, Rangwala SM, Keilbaugh SA, Flanigan A, Murthy S, Lazar MA, Wu GD. A novel therapy for colitis utilizing PPAR-{gamma} ligands to inhibit the epithelial inflammatory response. J Clin Invest. 1999;104(4):383–389. doi: 10.1172/JCI7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D, Fu L, Ning W, Guo L, Sun X, Dey SK, Chaturvedi R, Wilson KT, DuBois RN. Peroxisome proliferator-activated receptor δ promotes colonic inflammation and tumor growth. Proceedings of the National Academy of Sciences; 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhn H, O’Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Progress in Lipid Research. 2006;45(4):334–356. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, Colgan SP, Stahl GL, Merched A, Petasis NA, Chan L, Van Dyke TE. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171(12):6856–65. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- 26.Munger KA, Montero A, Fukunaga M, Uda S, Yura T, Imai E, Kaneda Y, Valdivielso JM, Badr KF. Transfection of rat kidney with human 15-lipoxygenase suppresses inflammation and preserves function in experimental glomerulonephritis. Proceedings of the National Academy of Sciences. 1999;96(23):13375–13380. doi: 10.1073/pnas.96.23.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki T, Fujii K, Yoshida K, Shimura H, Sasahira T, Ohmori H, Kuniyasu H. Peritoneal metastasis inhibition by linoleic acid with activation of PPARγ in human gastrointestinal cancer cells. Virchows Archiv. 2006;448(4):422–427. doi: 10.1007/s00428-005-0110-4. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka T, Kohno H, Yoshitani S-i, Takashima S, Okumura A, Murakami A, Hosokawa M. Ligands for Peroxisome Proliferator-activated Receptors {{alpha}} and {{gamma}} Inhibit Chemically Induced Colitis and Formation of Aberrant Crypt Foci in Rats1. Cancer Res. 2001;61(6):2424–2428. [PubMed] [Google Scholar]
- 29.Ligumsky M, Simon PL, Karmeli F, Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut. 1990;31(6):686–689. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawson MM, Thomas AG, Akobeng AK. Tumour necrosis factor alpha blocking agents for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006;3:CD005112. doi: 10.1002/14651858.CD005112.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Shureiqi I, Chen D, Day RS, Zuo X, Hochman FL, Ross WA, Cole RA, Moy O, Morris JS, Xiao L, Newman RA, Yang P, Lippman SM. Profiling lipoxygenase metabolism in specific steps of colorectal tumorigenesis. Cancer Prev Res (Phila) 2010;3(7):829–38. doi: 10.1158/1940-6207.CAPR-09-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuo X, Peng Z, Wu Y, Moussalli MJ, Yang XL, Wang Y, Parker-Thornburg J, Morris JS, Broaddus RR, Fischer SM, Shureiqi I. Effects of Gut-Targeted 15-LOX-1 Transgene Expression on Colonic Tumorigenesis in Mice. Journal of the National Cancer Institute. 2012;104(9):709–716. doi: 10.1093/jnci/djs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2(7):612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 34.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxin biosynthesis: an update and role in anti-inflammation and pro-resolution. Prostaglandins & Other Lipid Mediators. 2002;68–69:433–455. doi: 10.1016/s0090-6980(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 35.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eickmeier O, Seki H, Haworth O, Hilberath JN, Gao F, Uddin M, Croze RH, Carlo T, Pfeffer MA, Levy BD. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol. 2013;6(2):256–266. doi: 10.1038/mi.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. The Journal of Clinical Investigation. 2011;121(2):569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192(8):1197–204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serhan CN, Petasis NA. Resolvins and Protectins in Inflammation Resolution. Chemical Reviews. 2011;111(10):5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, Serhan CN, Kang JX. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci U S A. 2006;103(30):11276–81. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(21):7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bento AF, Claudino RF, Dutra RC, Marcon R, Calixto JB. Omega-3 Fatty Acid-Derived Mediators 17(R)-Hydroxy Docosahexaenoic Acid, Aspirin-Triggered Resolvin D1 and Resolvin D2 Prevent Experimental Colitis in Mice. The Journal of Immunology. 2011;187(4):1957–1969. doi: 10.4049/jimmunol.1101305. [DOI] [PubMed] [Google Scholar]
- 43.Ivanov I, Kuhn H, Heydeck D. Structural and functional biology of arachidonic acid 15-lipoxygenase-1 (ALOX15) Gene. 2015;573(1):1–32. doi: 10.1016/j.gene.2015.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ackermann JA, Hofheinz K, Zaiss MM, Kronke G. The double-edged role of 12/15-lipoxygenase during inflammation and immunity. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbalip.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 45.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schif-Zuck S, Gross N, Assi S, Rostoker R, Serhan CN, Ariel A. Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. Eur J Immunol. 2011;41(2):366–79. doi: 10.1002/eji.201040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281(50):38376–84. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- 49.Ariel A, Serhan CN. New Lives Given by Cell Death: Macrophage Differentiation Following Their Encounter with Apoptotic Leukocytes during the Resolution of Inflammation. Front Immunol. 2012;3:4. doi: 10.3389/fimmu.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bystrom J, Evans I, Newson J, Stables M, Toor I, van Rooijen N, Crawford M, Colville-Nash P, Farrow S, Gilroy DW. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood. 2008;112(10):4117–27. doi: 10.1182/blood-2007-12-129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120(15):e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stables MJ, Shah S, Camon EB, Lovering RC, Newson J, Bystrom J, Farrow S, Gilroy DW. Transcriptomic analyses of murine resolution-phase macrophages. Blood. 2011;118(26):e192–208. doi: 10.1182/blood-2011-04-345330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conrad DJ, Kuhn H, Mulkins M, Highland E, Sigal E. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc Natl Acad Sci U S A. 1992;89(1):217–21. doi: 10.1073/pnas.89.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heydeck D, Thomas L, Schnurr K, Trebus F, Thierfelder WE, Ihle JN, Kuhn H. Interleukin-4 and -13 Induce Upregulation of the Murine Macrophage 12/15-Lipoxygenase Activity: Evidence for the Involvement of Transcription Factor STAT6. Blood. 1998;92(7):2503–2510. [PubMed] [Google Scholar]
- 55.Rostoker R, Yaseen H, Schif-Zuck S, Lichtenstein RG, Rabinovich GA, Ariel A. Galectin-1 induces 12/15-lipoxygenase expression in murine macrophages and favors their conversion toward a pro-resolving phenotype. Prostaglandins Other Lipid Mediat. 2013;107:85–94. doi: 10.1016/j.prostaglandins.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Uderhardt S, Herrmann M, Oskolkova OV, Aschermann S, Bicker W, Ipseiz N, Sarter K, Frey B, Rothe T, Voll R, Nimmerjahn F, Bochkov VN, Schett G, Kronke G. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity. 2012;36(5):834–46. doi: 10.1016/j.immuni.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 57.Hunter MM, Wang A, Parhar KS, Johnston MJ, Van Rooijen N, Beck PL, McKay DM. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138(4):1395–405. doi: 10.1053/j.gastro.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 58.Engelhardt KR, Grimbacher B. IL-10 in humans: lessons from the gut, IL-10/IL-10 receptor deficiencies, and IL-10 polymorphisms. Curr Top Microbiol Immunol. 2014;380:1–18. doi: 10.1007/978-3-662-43492-5_1. [DOI] [PubMed] [Google Scholar]
- 59.Wang K, Karin M. Tumor-Elicited Inflammation and Colorectal Cancer. Adv Cancer Res. 2015;128:173–96. doi: 10.1016/bs.acr.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 60.Zigmond E, Bernshtein B, Friedlander G, Walker CR, Yona S, Kim KW, Brenner O, Krauthgamer R, Varol C, Muller W, Jung S. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity. 2014;40(5):720–33. doi: 10.1016/j.immuni.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 61.Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS, Mascanfroni ID, Al Adham Z, Lavoie S, Ibourk M, Nguyen DD, Samsom JN, Escher JC, Somech R, Weiss B, Beier R, Conklin LS, Ebens CL, Santos FG, Ferreira AR, Sherlock M, Bhan AK, Muller W, Mora JR, Quintana FJ, Klein C, Muise AM, Horwitz BH, Snapper SB. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40(5):706–19. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fredman G, Li Y, Dalli J, Chiang N, Serhan CN. Self-limited versus delayed resolution of acute inflammation: temporal regulation of pro-resolving mediators and microRNA. Sci Rep. 2012;2:639. doi: 10.1038/srep00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Titos E, Rius B, González-Périz A, López-Vicario C, Morán-Salvador E, Martínez-Clemente M, Arroyo V, Clària J. Resolvin D1 and Its Precursor Docosahexaenoic Acid Promote Resolution of Adipose Tissue Inflammation by Eliciting Macrophage Polarization toward an M2-Like Phenotype. The Journal of Immunology. 2011;187(10):5408–5418. doi: 10.4049/jimmunol.1100225. [DOI] [PubMed] [Google Scholar]
- 64.Gross M, Salame TM, Jung S. Guardians of the Gut - Murine Intestinal Macrophages and Dendritic Cells. Front Immunol. 2015;6:254. doi: 10.3389/fimmu.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Isidro RA, Appleyard CB. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol. 2016;311(1):G59–73. doi: 10.1152/ajpgi.00123.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Erreni M, Mantovani A, Allavena P. Tumor-associated Macrophages (TAM) and Inflammation in Colorectal Cancer. Cancer Microenviron. 2011;4(2):141–54. doi: 10.1007/s12307-010-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norton SE, Dunn ET, McCall JL, Munro F, Kemp RA. Gut macrophage phenotype is dependent on the tumor microenvironment in colorectal cancer. Clin Transl Immunology. 2016;5(4):e76. doi: 10.1038/cti.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shureiqi I, Wojno KJ, Poore JA, Reddy RG, Moussalli MJ, Spindler SA, Greenson JK, Normolle D, Hasan AA, Lawrence TS, Brenner DE. Decreased 13-S-hydroxyoctadecadienoic acid levels and 15-lipoxygenase-1 expression in human colon cancers. Carcinogenesis. 1999;20(10):1985–95. doi: 10.1093/carcin/20.10.1985. [DOI] [PubMed] [Google Scholar]
- 70.Nixon JB, Kim KS, Lamb PW, Bottone FG, Eling TE. 15-Lipoxygenase-1 has anti-tumorigenic effects in colorectal cancer. Prostaglandins Leukot Essent Fatty Acids. 2004;70(1):7–15. doi: 10.1016/j.plefa.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 71.Heslin MJ, Hawkins A, Boedefeld W, Arnoletti JP, Frolov A, Soong R, Urist MM, Bland KI. Tumor-associated down-regulation of 15-lipoxygenase-1 is reversed by celecoxib in colorectal cancer. Ann Surg. 2005;241(6):941–6. doi: 10.1097/01.sla.0000164177.95620.c1. discussion 946–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuri M, Sasahira T, Nakai K, Ishimaru S, Ohmori H, Kuniyasu H. Reversal of expression of 15-lipoxygenase-1 to cyclooxygenase-2 is associated with development of colonic cancer. Histopathology. 2007;51(4):520–527. doi: 10.1111/j.1365-2559.2007.02799.x. [DOI] [PubMed] [Google Scholar]
- 73.Shureiqi I, Wu Y, Chen D, Yang XL, Guan B, Morris JS, Yang P, Newman RA, Broaddus R, Hamilton SR, Lynch P, Levin B, Fischer SM, Lippman SM. The Critical Role of 15-Lipoxygenase-1 in Colorectal Epithelial Cell Terminal Differentiation and Tumorigenesis. Cancer Res. 2005;65(24):11486–11492. doi: 10.1158/0008-5472.CAN-05-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zuo X, Shureiqi I. Eicosanoid profiling in colon cancer: Emergence of a pattern. Prostaglandins & Other Lipid Mediators. 2012 doi: 10.1016/j.prostaglandins.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moussalli MJ, Wu Y, Zuo X, Yang XL, Wistuba, Raso MG, Morris JS, Bowser JL, Minna JD, Lotan R, Shureiqi I. Mechanistic contribution of ubiquitous 15-lipoxygenase-1 expression loss in cancer cells to terminal cell differentiation evasion. Cancer Prev Res (Phila) 2011;4(12):1961–72. doi: 10.1158/1940-6207.CAPR-10-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shureiqi I, Xu X, Chen D, Lotan R, Morris JS, Fischer SM, Lippman SM. Nonsteroidal anti-inflammatory drugs induce apoptosis in esophageal cancer cells by restoring 15-lipoxygenase-1 expression. Cancer Research. 2001;61(12):4879–84. [PubMed] [Google Scholar]
- 77.Jiang WG, Watkins G, Douglas-Jones A, Mansel RE. Reduction of isoforms of 15-lipoxygenase (15-LOX)-1 and 15-LOX-2 in human breast cancer, Prostaglandins. Leukotrienes and Essential Fatty Acids. 2006;74(4):235–245. doi: 10.1016/j.plefa.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 78.Sak ME, Alanbay I, Rodriguez A, Gokaslan T, Borahay M, Shureiqi I, Kilic GS. The role of 15-lipoxygenase-1 expression and its potential role in the pathogenesis of endometrial hyperplasia and endometrial adenocarcinomas. Eur J Gynaecol Oncol. 2016;37(1):36–40. [PubMed] [Google Scholar]
- 79.Philips BJ, Dhir R, Hutzley J, Sen M, Kelavkar UP. Polyunsaturated fatty acid metabolizing 15-Lipoxygenase-1 (15-LO-1) expression in normal and tumorigenic human bladder tissues. Appl Immunohistochem Mol Morphol. 2008;16(2):159–64. doi: 10.1097/PAI.0b013e31805baa41. [DOI] [PubMed] [Google Scholar]
- 80.Hennig R, Kehl T, Noor S, Ding XZ, Rao SM, Bergmann F, Furstenberger G, Buchler MW, Friess H, Krieg P, Adrian TE. 15-Lipoxygenase-1 Production is Lost in Pancreatic Cancer and Overexpression of the Gene Inhibits Tumor Cell Growth. Neoplasia. 2007;9(11):917–26. doi: 10.1593/neo.07565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zuo X, Morris JS, Broaddus R, Shureiqi I. 15-LOX-1 transcription suppression through the NuRD complex in colon cancer cells. Oncogene. 2009;28(12):1496–1505. doi: 10.1038/onc.2008.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mao F, Xu M, Zuo X, Yu J, Xu W, Moussalli MJ, Elias E, Li HS, Watowich SS, Shureiqi I. 15-Lipoxygenase-1 suppression of colitis-associated colon cancer through inhibition of the IL-6/STAT3 signaling pathway. The FASEB Journal. 2015;29(6):2359–2370. doi: 10.1096/fj.14-264515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Il Lee S, Zuo X, Shureiqi I. 15-Lipoxygenase-1 as a tumor suppressor gene in colon cancer: is the verdict in? Cancer and Metastasis Reviews. 2011;30(3):481–491. doi: 10.1007/s10555-011-9321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shureiqi I, Chen D, Lee JJ, Yang P, Newman RA, Brenner DE, Lotan R, Fischer SM, Lippman SM. 15-LOX-1: a novel molecular target of nonsteroidal anti-inflammatory drug-induced apoptosis in colorectal cancer cells. J Natl Cancer Inst. 2000;92(14):1136–42. doi: 10.1093/jnci/92.14.1136. [DOI] [PubMed] [Google Scholar]
- 85.Deguchi A, Xing SW, Shureiqi I, Yang P, Newman RA, Lippman SM. Activation of protein kinase G up-regulates expression of 15-lipoxygenase-1 in human colon cancer cells. Cancer Research. 2005;65:8442–8447. doi: 10.1158/0008-5472.CAN-05-1109. [DOI] [PubMed] [Google Scholar]
- 86.Wu Y, Fang B, Yang XQ, Wang L, Chen D, Krasnykh V, Carter BZ, Morris JS, Shureiqi I. Therapeutic Molecular Targetingof 15-Lipoxygenase-1 in Colon Cancer. Mol Ther. 2008;16(5):886–892. doi: 10.1038/mt.2008.44. [DOI] [PubMed] [Google Scholar]
- 87.Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Desvergne B, Wahli W. Peroxisome Proliferator-Activated Receptors: Nuclear Control of Metabolism. Endocrine Reviews. 1999;20(5):649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 89.Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2011;1812(8):1007–1022. doi: 10.1016/j.bbadis.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu M, Zuo X, Shureiqi I. Targeting peroxisome proliferator-activated receptor-β/δ in colon cancer: How to aim? Biochemical Pharmacology. 2013;85(5):607–611. doi: 10.1016/j.bcp.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zuo X, Xu M, Yu J, Wu Y, Moussalli MJ, Manyam GC, Lee SI, Liang S, Gagea M, Morris JS, Broaddus RR, Shureiqi I. Potentiation of colon cancer susceptibility in mice by colonic epithelial PPAR-delta/beta overexpression. J Natl Cancer Inst. 2014;106(4):dju052. doi: 10.1093/jnci/dju052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong SJ, Bauer-Rowe KE, Xifaras ME, Akkad A, Arias E, Pinello L, Katz Y, Shinagare S, Abu-Remaileh M, Mihaylova MM, Lamming DW, Dogum R, Guo G, Bell GW, Selig M, Nielsen GP, Gupta N, Ferrone CR, Deshpande V, Yuan GC, Orkin SH, Sabatini DM, Yilmaz OH. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature. 2016;531(7592):53–8. doi: 10.1038/nature17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Harman FS, Nicol CJ, Marin HE, Ward JM, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-delta attenuates colon carcinogenesis. Nature Medicine. 2004;10(5):481–483. doi: 10.1038/nm1026. [DOI] [PubMed] [Google Scholar]
- 94.Wang D, Wang H, Guo Y, Ning W, Katkuri S, Wahli W, Desvergne B, Dey SK, DuBois RN. Crosstalk between peroxisome proliferator-activated receptor delta and VEGF stimulates cancer progression. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(50):19069–74. doi: 10.1073/pnas.0607948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zuo X, Peng Z, Moussalli MJ, Morris JS, Broaddus RR, Fischer SM, Shureiqi I. Targeted Genetic Disruption of Peroxisome Proliferator-Activated Receptor-{delta} and Colonic Tumorigenesis. J Natl Cancer Inst. 2009;101(10):762–767. doi: 10.1093/jnci/djp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takahashi H, Jin C, Rajabi H, Pitroda S, Alam M, Ahmad R, Raina D, Hasegawa M, Suzuki Y, Tagde A, Bronson RT, Weichselbaum R, Kufe D. MUC1-C activates the TAK1 inflammatory pathway in colon cancer. Oncogene. 2015;34(40):5187–5197. doi: 10.1038/onc.2014.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beatty PL, Plevy SE, Sepulveda AR, Finn OJ. Cutting Edge: Transgenic Expression of Human MUC1 in IL-10/Mice Accelerates Inflammatory Bowel Disease and Progression to Colon Cancer. The Journal of Immunology. 2007;179(2):735–739. doi: 10.4049/jimmunol.179.2.735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.