Abstract
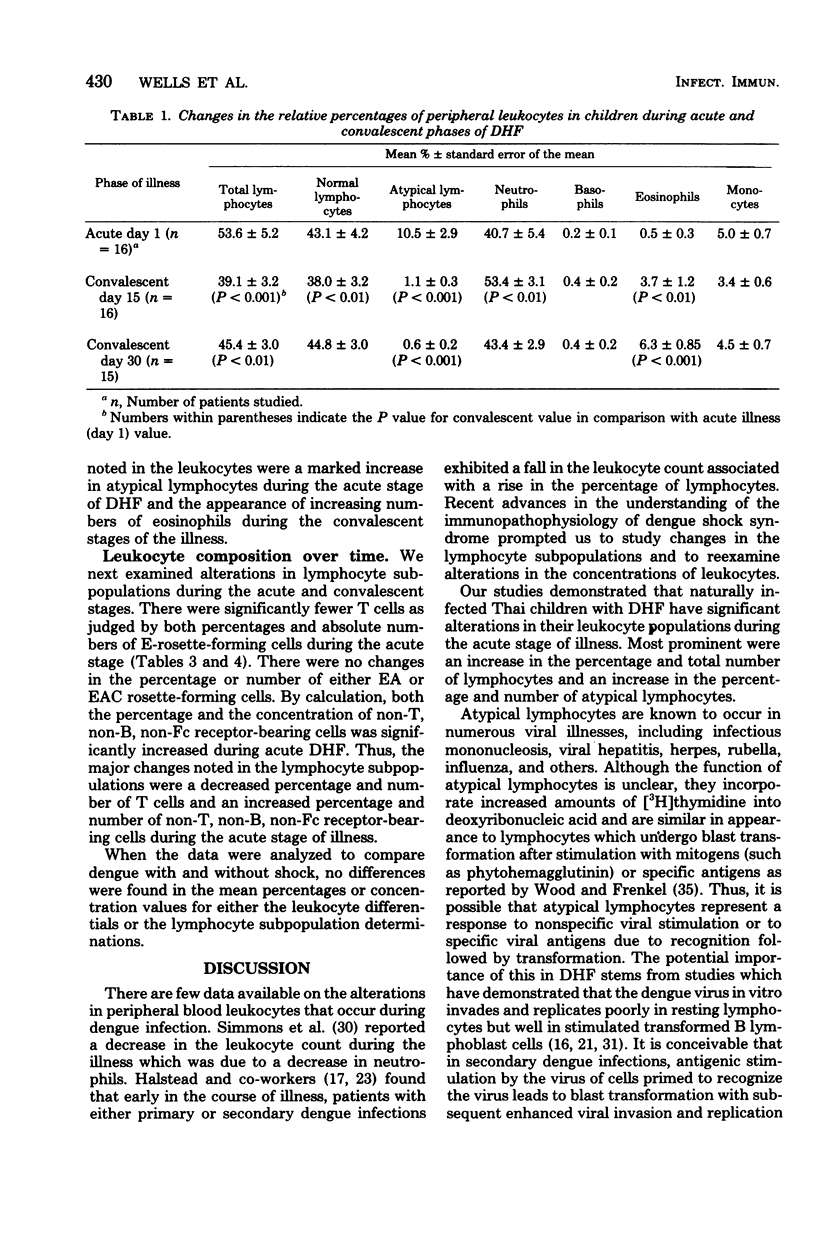
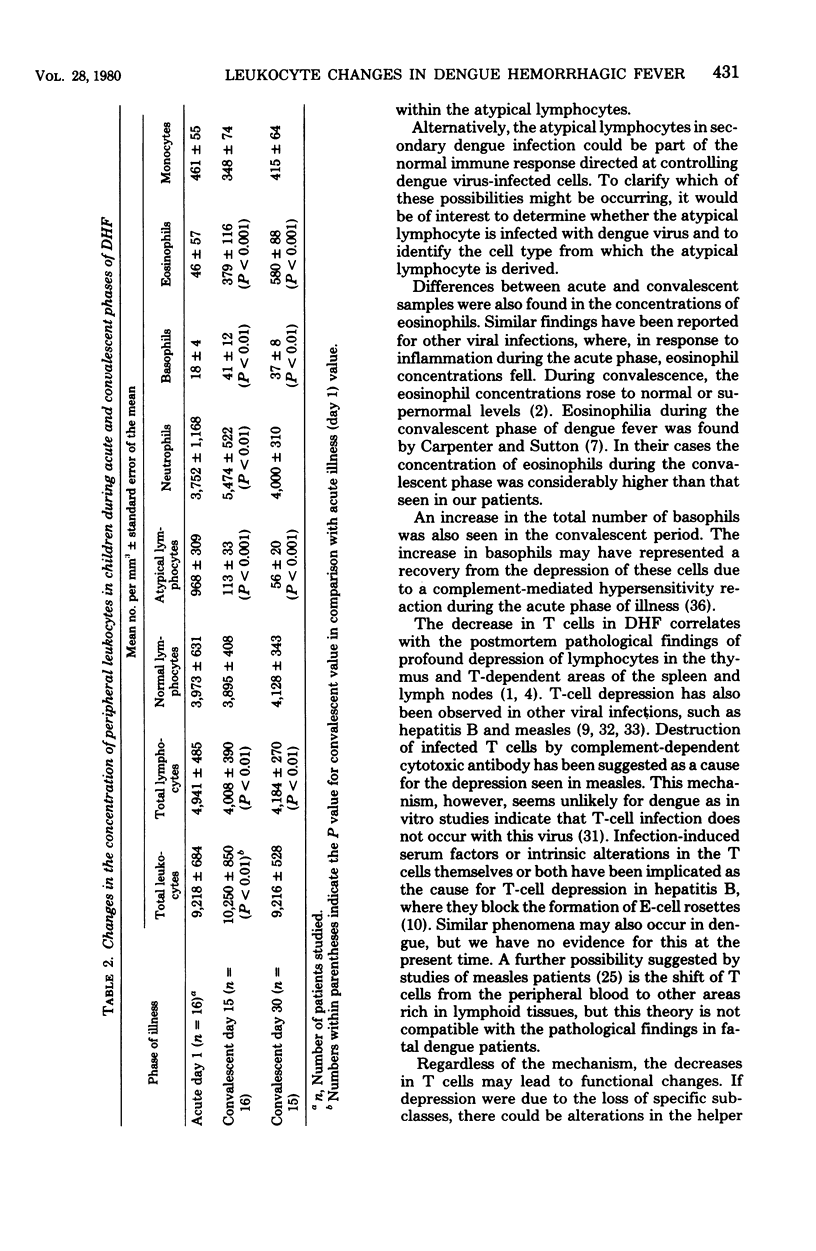
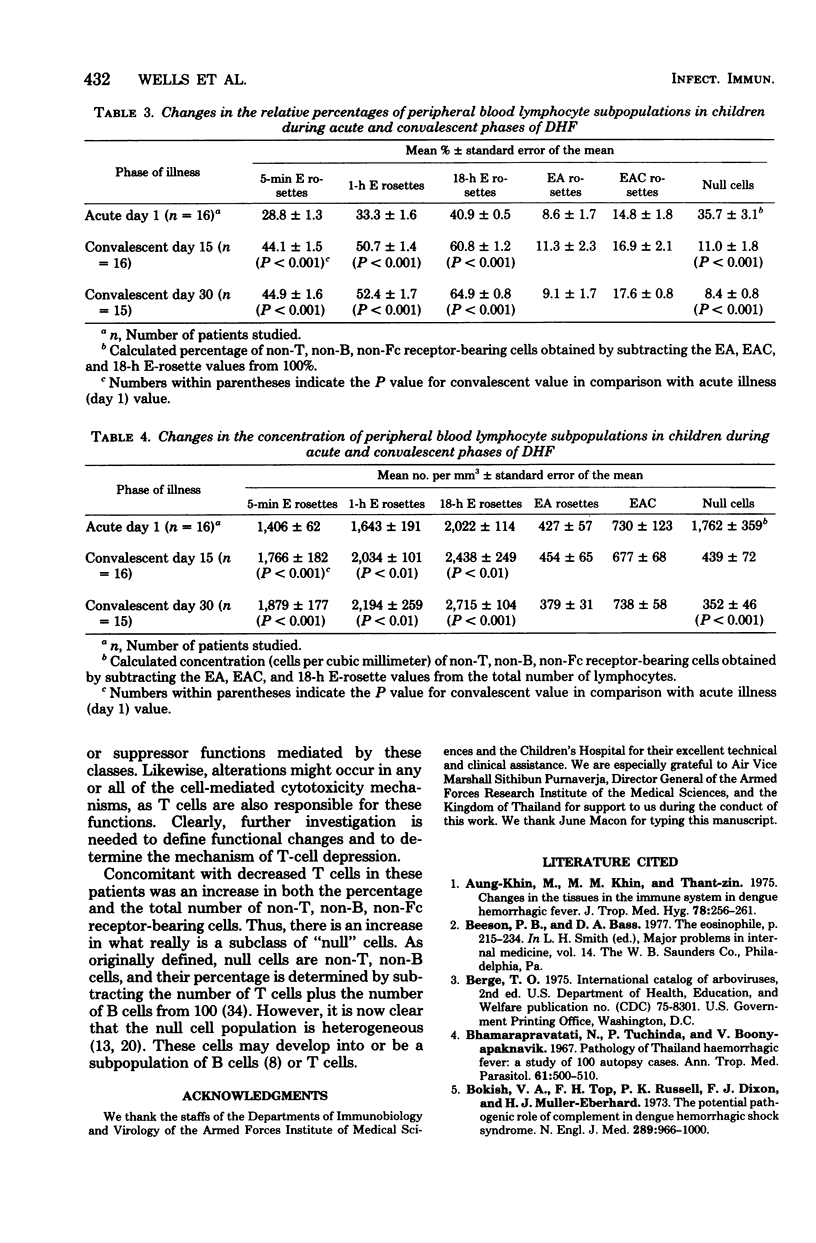
Peripheral leukocytes from 16 Thai children with dengue hemorrhagic fever were examined to determine the leukocyte composition on the day of presentation and on convalescent days 15 and 30. Mononuclear cells were isolated each time, and the concentrations of T, B, Fc receptor-bearing, and "null" cells were determined. On the day of hospitalization, in comparison to convalescent values, there was a significant increase in total lymphocytes, primarily due to concentrations of atypical lymphocytes. There was a significant loss of T cells with an increase in non-T, non-B, non-Fc receptor-bearing null cells. There were no changes in the concentrations of monocytes, B cells, or Fc receptor-bearing cells when acute and convalescent values were compared. During the convalescent period, a progressive increase in eosinophils was noted. Also, on day 15 but not on day 30 of the convalescent period, an increase was observed in the total leukocyte number due to an increase in granulocytes. There results indicate that in Thai children with dengue hemorrhagic fever, there are major shifts within several component cell subpopulations of the immune system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aung-Khin M., Ma-Ma K., Thant-Zin Changes in the tissues of the immune system in dengue haemorrhagic fever. J Trop Med Hyg. 1975 Dec;78(12):256–261. [PubMed] [Google Scholar]
- Bhamarapravati N., Tuchinda P., Boonyapaknavik V. Pathology of Thailand haemorrhagic fever: a study of 100 autopsy cases. Ann Trop Med Parasitol. 1967 Dec;61(4):500–510. doi: 10.1080/00034983.1967.11686519. [DOI] [PubMed] [Google Scholar]
- Bokisch V. A., Top F. H., Jr, Russell P. K., Dixon F. J., Müller-Eberhard H. J. The potential pathogenic role of complement in dengue hemorrhagic shock syndrome. N Engl J Med. 1973 Nov 8;289(19):996–1000. doi: 10.1056/NEJM197311082891902. [DOI] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. I. Quantitative isolation of human T and B cells and response to mitogens. J Immunol. 1974 Oct;113(4):1113–1121. [PubMed] [Google Scholar]
- Chisari F. V., Edgington T. S. Lymphocyte E rosette inhibitory factor: a regulatory serum lipoprotein. J Exp Med. 1975 Nov 1;142(5):1092–1107. doi: 10.1084/jem.142.5.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari F. V., Routenberg J. A., Fiala M., Edgington T. S. Extrinsic modulation of human T-lymphocyte E rosette function associated with prolonged hepatocellular injury after viral hepatitis. J Clin Invest. 1977 Jan;59(1):134–142. doi: 10.1172/JCI108610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Halstead S. B. Shock associated with dengue infection. I. Clinical and physiologic manifestations of dengue hemorrhagic fever in Thailand, 1964. J Pediatr. 1966 Mar;68(3):448–456. doi: 10.1016/s0022-3476(66)80249-4. [DOI] [PubMed] [Google Scholar]
- Froland S. S., Natvig J. B. Identification of three different human lymphocyte populations by surface markers. Transplant Rev. 1973;16:114–162. doi: 10.1111/j.1600-065x.1973.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., Chow J. S., Marchette N. J. Immunological enhancement of dengue virus replication. Nat New Biol. 1973 May 2;243(122):24–26. [PubMed] [Google Scholar]
- Halstead S. B. Mosquito-borne haemorrhagic fevers of South and South-East Asia. Bull World Health Organ. 1966;35(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., Nimmannitya S., Cohen S. N. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J Biol Med. 1970 Apr;42(5):311–328. [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J., Allison A. C. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J Exp Med. 1977 Jul 1;146(1):218–229. doi: 10.1084/jem.146.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977 Jul 1;146(1):201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B. Observations related to pathogensis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale J Biol Med. 1970 Apr;42(5):350–362. [PMC free article] [PubMed] [Google Scholar]
- Horwitz D. A., Lobo P. I. Characterizaiton of two populations of human lymphocytes bearing easily detectable surface immunoglobulin. J Clin Invest. 1975 Dec;56(6):1464–1472. doi: 10.1172/JCI108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchette N. J., Halstead S. B. Phytohemagglutinin enhancement of dengue-2 virus replication in nonimmune rhesus monkey peripheral blood leukocytes. Infect Immun. 1978 Jan;19(1):40–45. doi: 10.1128/iai.19.1.40-45.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmannitya S., Halstead S. B., Cohen S. N., Margiotta M. R. Dengue and chikungunya virus infection in man in Thailand, 1962-1964. I. Observations on hospitalized patients with hemorrhagic fever. Am J Trop Med Hyg. 1969 Nov;18(6):954–971. doi: 10.4269/ajtmh.1969.18.954. [DOI] [PubMed] [Google Scholar]
- Peter H. H., Pavie-Fischer J., Fridman W. H., Aubert C., Cesarini J. P., Roubin R., Kourilsky F. M. Cell-mediate cytotoxicity in vitro of human lymphocytes against a tissue culture melanoma cell line (igr3). J Immunol. 1975 Aug;115(2):539–548. [PubMed] [Google Scholar]
- Rook G. A., Carswell J. W., Stanford J. L. Preliminary evidence for the trapping of antigen-specific lymphocytes in the lymphoid tissue of 'anergic' tuberculosis patients. Clin Exp Immunol. 1976 Oct;26(1):129–132. [PMC free article] [PubMed] [Google Scholar]
- Russell P. K., Nisalak A. Dengue virus identification by the plaque reduction neutralization test. J Immunol. 1967 Aug;99(2):291–296. [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Scott R. M., Nimmannitya S., Bancroft W. H., Mansuwan P. Shock syndrome in primary dengue infections. Am J Trop Med Hyg. 1976 Nov;25(6):866–874. doi: 10.4269/ajtmh.1976.25.866. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Brandt W. E., Russell P. K., Dixon F. T. Replication of dengue-2 virus in cultured human lymphoblastoid cells and subpopulations of human peripheral leukocytes. J Immunol. 1976 Sep;117(3):953–961. [PubMed] [Google Scholar]
- Wesley A., Coovadia H. M., Henderson L. Immunological recovery after measles. Clin Exp Immunol. 1978 Jun;32(3):540–544. [PMC free article] [PubMed] [Google Scholar]
- Whittle H. C., Dossetor J., Oduloju A., Bryceson A. D., Greenwood B. M. Cell-mediated immunity during natural measles infection. J Clin Invest. 1978 Sep;62(3):678–684. doi: 10.1172/JCI109175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, DeBoard J. R., Mellbye O. J., Messner R. P., Lindström F. D. Studies of T- and B-lymphocytes in patients with connective tissue diseases. J Clin Invest. 1973 Feb;52(2):283–295. doi: 10.1172/JCI107184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. A., Frenkel E. P. The atypical lymphocyte. Am J Med. 1967 Jun;42(6):923–936. doi: 10.1016/0002-9343(67)90073-3. [DOI] [PubMed] [Google Scholar]
- Yuill T. M., Sukhavachana P., Nisalak A., Russell P. K. Dengue-virus recovery by direct and delayed plaques in LLC-MK2 cells. Am J Trop Med Hyg. 1968 May;17(3):441–448. doi: 10.4269/ajtmh.1968.17.441. [DOI] [PubMed] [Google Scholar]


