Abstract
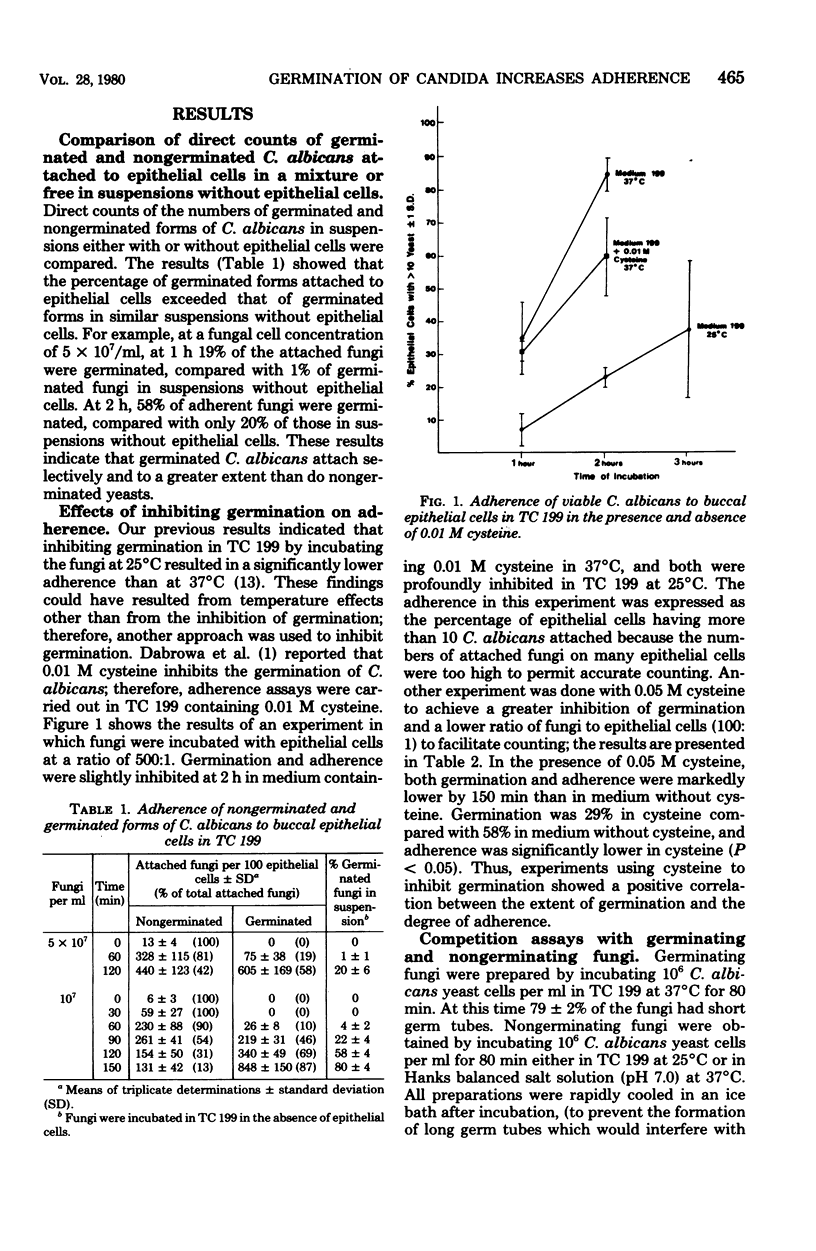
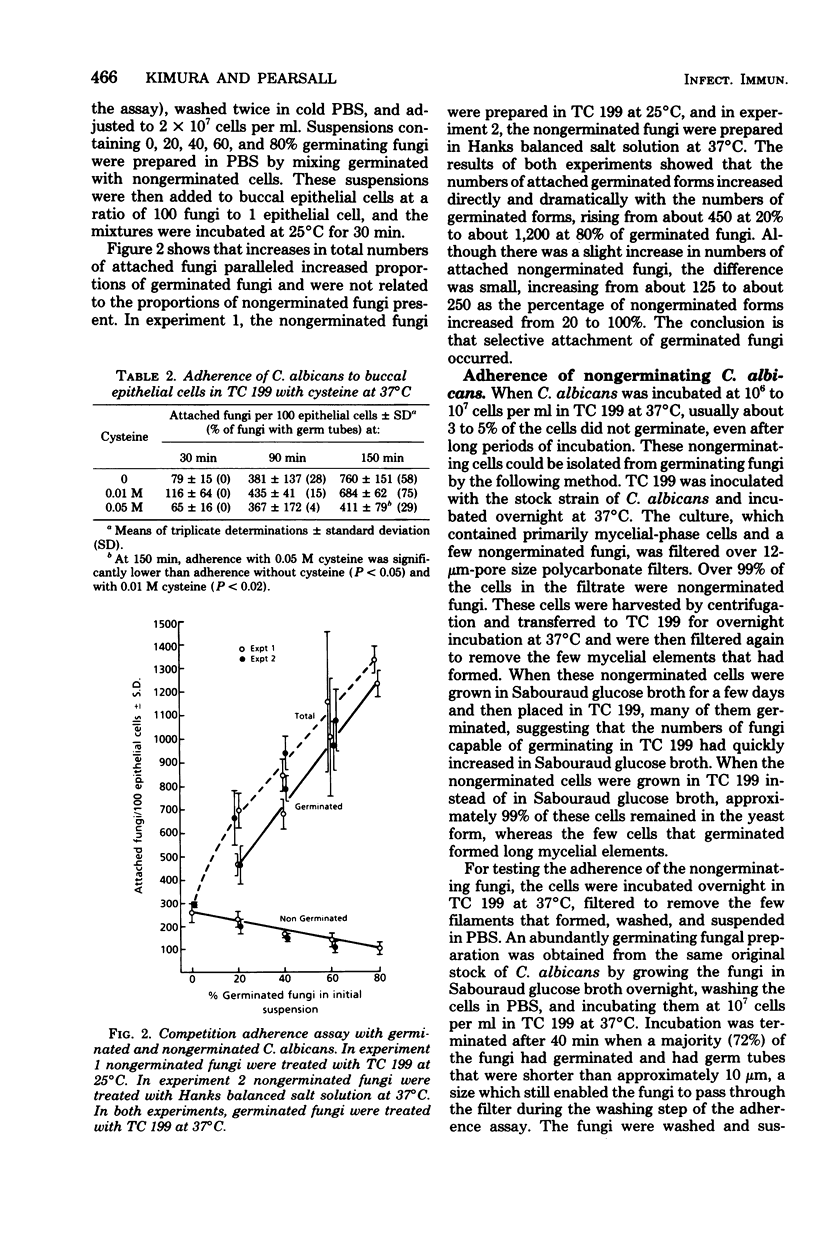
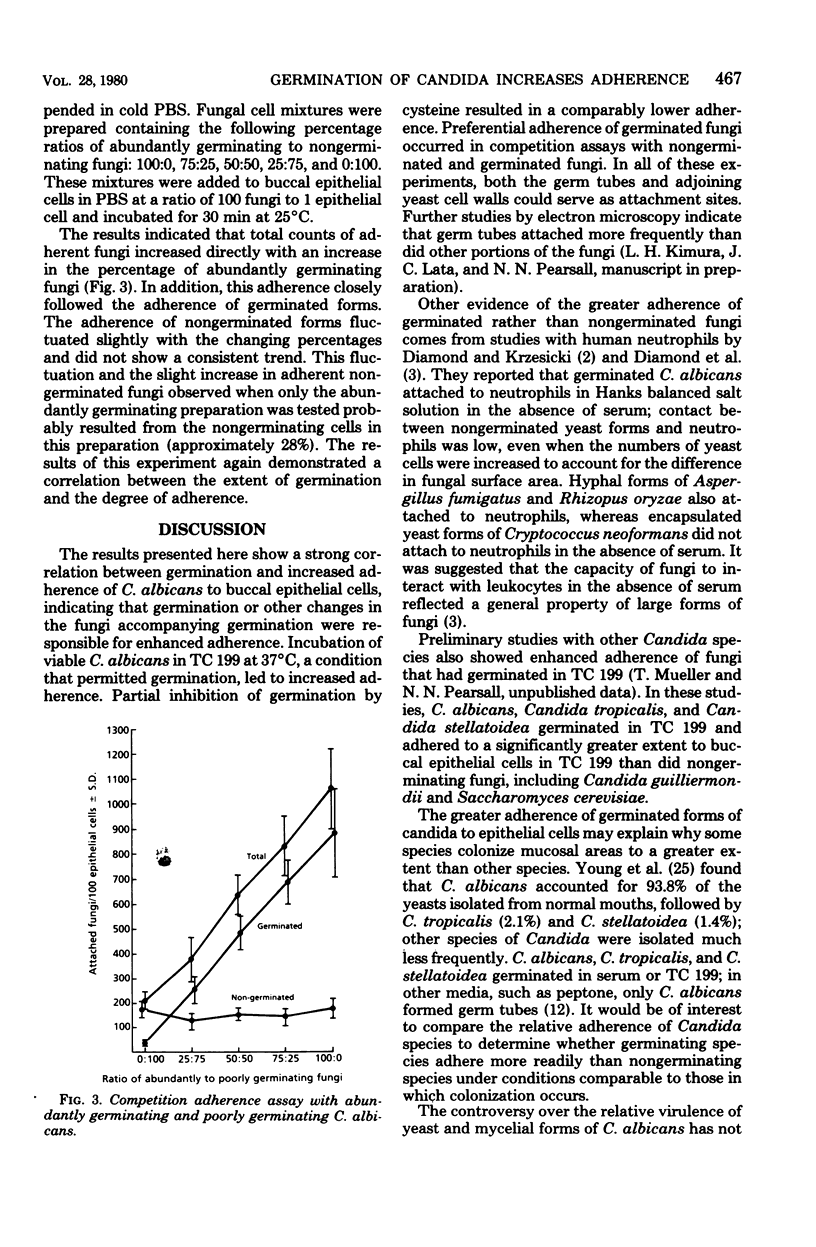
A strong correlation was shown between germination and increased adherence of Candida albicans to human buccal epithelial cells, indicating that germination or other changes in the fungi accompanying germination were responsible for enhanced adherence. Partial inhibition of germination by cysteine resulted in a comparably lower adherence. Preferential adherence of germinated fungi occurred in competition assays with nongerminated and germinated fungi. The enhanced adherence to human mucosal cells of germinated C albicans could represent one mechanism contributing to the pathogenicity of the organism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dabrowa N., Taxer S. S., Howard D. H. Germination of Candida albicans induced by proline. Infect Immun. 1976 Mar;13(3):830–835. doi: 10.1128/iai.13.3.830-835.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daimond R. D., Krzesicki R. Mechanisms of attachment of neutrophils to Candida albicans pseudohyphae in the absence of serum, and of subsequent damage to pseudohyphae by microbicidal processes of neutrophils in vitro. J Clin Invest. 1978 Feb;61(2):360–369. doi: 10.1172/JCI108946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978 Feb;61(2):349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISMAN P. C., GEFTIC S. G., MAYER R. L. Virulence in mice of colonial variants of Candida albicans. Proc Soc Exp Biol Med. 1953 Feb;82(2):263–264. doi: 10.3181/00379727-82-20086. [DOI] [PubMed] [Google Scholar]
- Evans Z. A., Mardon D. N. Organ localization in mice challenged with a typical Candida albicans strain and a pseudohyphal variant. Proc Soc Exp Biol Med. 1977 Jun;155(2):234–238. doi: 10.3181/00379727-155-39780. [DOI] [PubMed] [Google Scholar]
- Freter R., Jones G. W. Adhesive properties of Vibrio cholerae: nature of the interaction with intact mucosal surfaces. Infect Immun. 1976 Jul;14(1):246–256. doi: 10.1128/iai.14.1.246-256.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEBHARDT L. P., HILL D. W. Morphological transformation of Candida albicans in tissues of mice. Proc Soc Exp Biol Med. 1956 Jul;92(3):640–644. doi: 10.3181/00379727-92-22570. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannini P. B., Arai G. D., LaForce F. M. Vascular clearance of blastospore and pseudomycelial phase Candida albicans. Sabouraudia. 1977 Jul;15(2):201–205. [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi K. R., Gavin J. B., Bremner D. A. The formation of germ tubes by Candida albicans in various peptone media. Sabouraudia. 1973 Nov;11(3):259–262. [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Adherence of Candida albicans to human buccal epithelial cells. Infect Immun. 1978 Jul;21(1):64–68. doi: 10.1128/iai.21.1.64-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Suppression of Candida albicans by human oral streptococci in gnotobiotic mice. Infect Immun. 1973 Nov;8(5):846–849. doi: 10.1128/iai.8.5.846-849.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardon D. N., Gunn J. L., Robinette E., Jr Variation in the lethal response in mice to yeast-like and pseudohyphal forms of Candida albicans. Can J Microbiol. 1975 Nov;21(11):1681–1687. doi: 10.1139/m75-246. [DOI] [PubMed] [Google Scholar]
- Mårdh P. A., Westtöm L. Adherence of bacterial to vaginal epithelial cells. Infect Immun. 1976 Mar;13(3):661–666. doi: 10.1128/iai.13.3.661-666.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearsall N. N., Lagunoff D. Immunological responses to Candida albicans. I. Mouse-thigh lesion as a model for experimental candidiasis. Infect Immun. 1974 Jun;9(6):999–1002. doi: 10.1128/iai.9.6.999-1002.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C., Jones J. H. The effects of oral inoculation of the yeast and mycelial phases of Candida albicans in rats fed on normal and carbohydrate rich diets. Arch Oral Biol. 1973 Mar;18(3):409–412. doi: 10.1016/0003-9969(73)90165-9. [DOI] [PubMed] [Google Scholar]
- Simonetti N., Strippoli V. Pathogenicity of the Y form as compared to M form in experimentally induced Candida albicans infections. Mycopathol Mycol Appl. 1973 Sep 28;51(1):19–28. doi: 10.1007/BF02141282. [DOI] [PubMed] [Google Scholar]
- WINSTEN S., MURRAY T. J. Virulence enhancement of a filamentous strain of Candida albicans after growth on media containing cysteine. J Bacteriol. 1956 Jun;71(6):738–738. doi: 10.1128/jb.71.6.738-738.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG G., RESCA H. G., SULLIVAN M. T. The yeasts of the normal mouth and their relation to salivary acidity. J Dent Res. 1951 Jun;30(3):426–430. doi: 10.1177/00220345510300031901. [DOI] [PubMed] [Google Scholar]
- YOUNG G. The process of invasion and the persistence of Candida albicans injected intraperitoneally into mice. J Infect Dis. 1958 Mar-Apr;102(2):114–120. doi: 10.1093/infdis/102.2.114. [DOI] [PubMed] [Google Scholar]


