Abstract
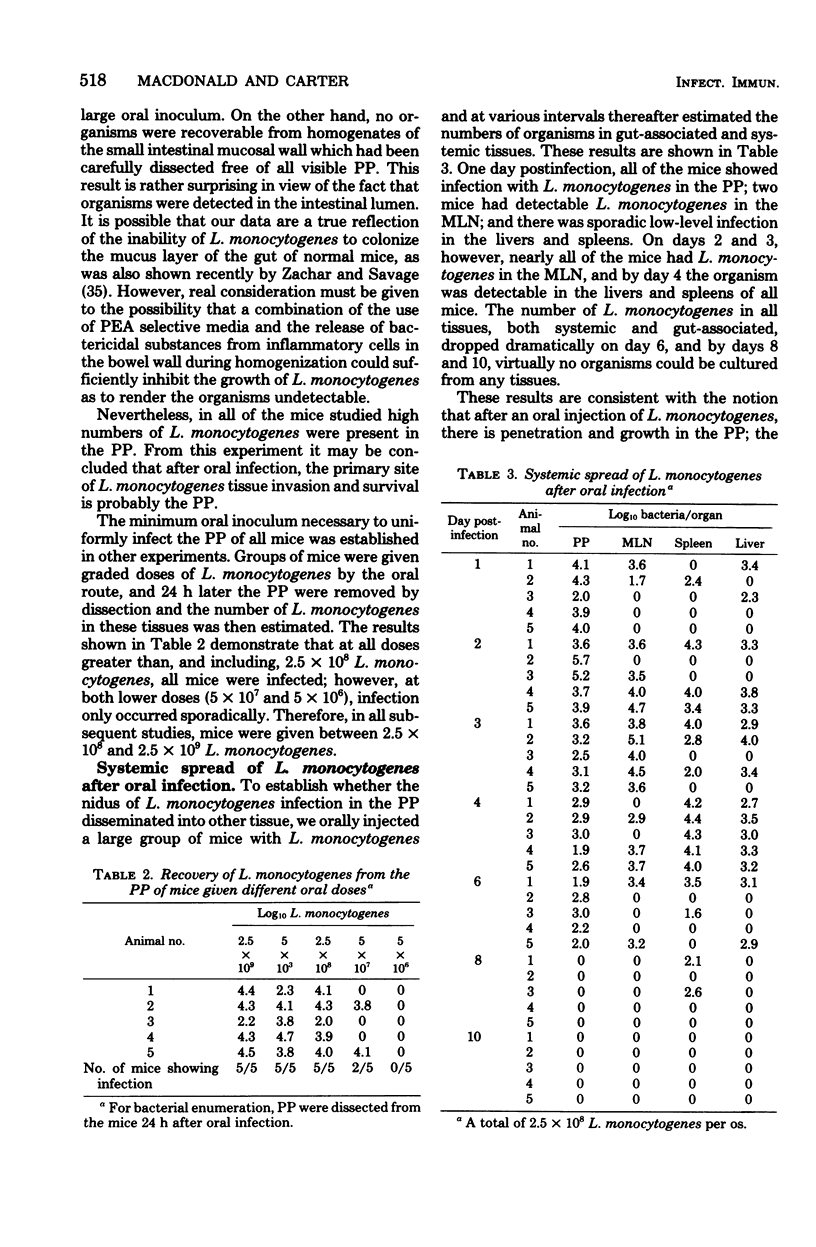
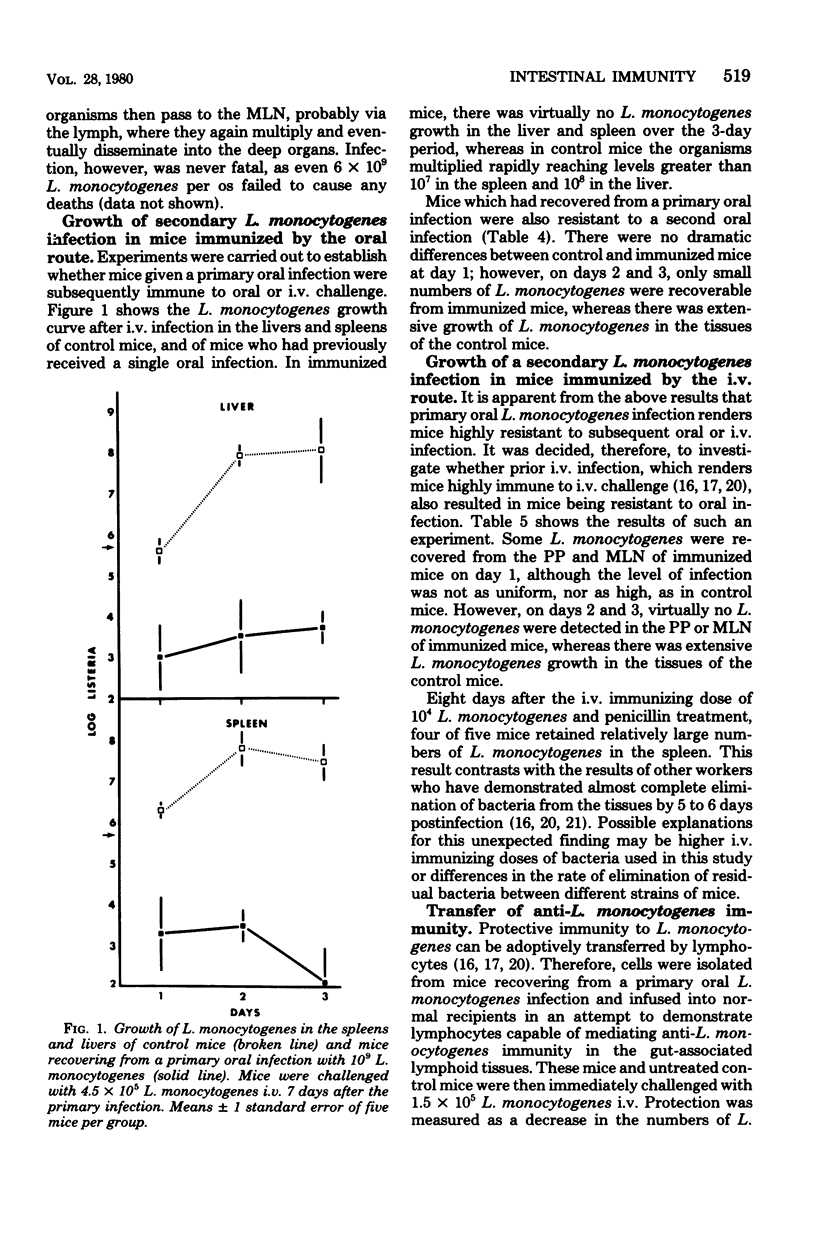
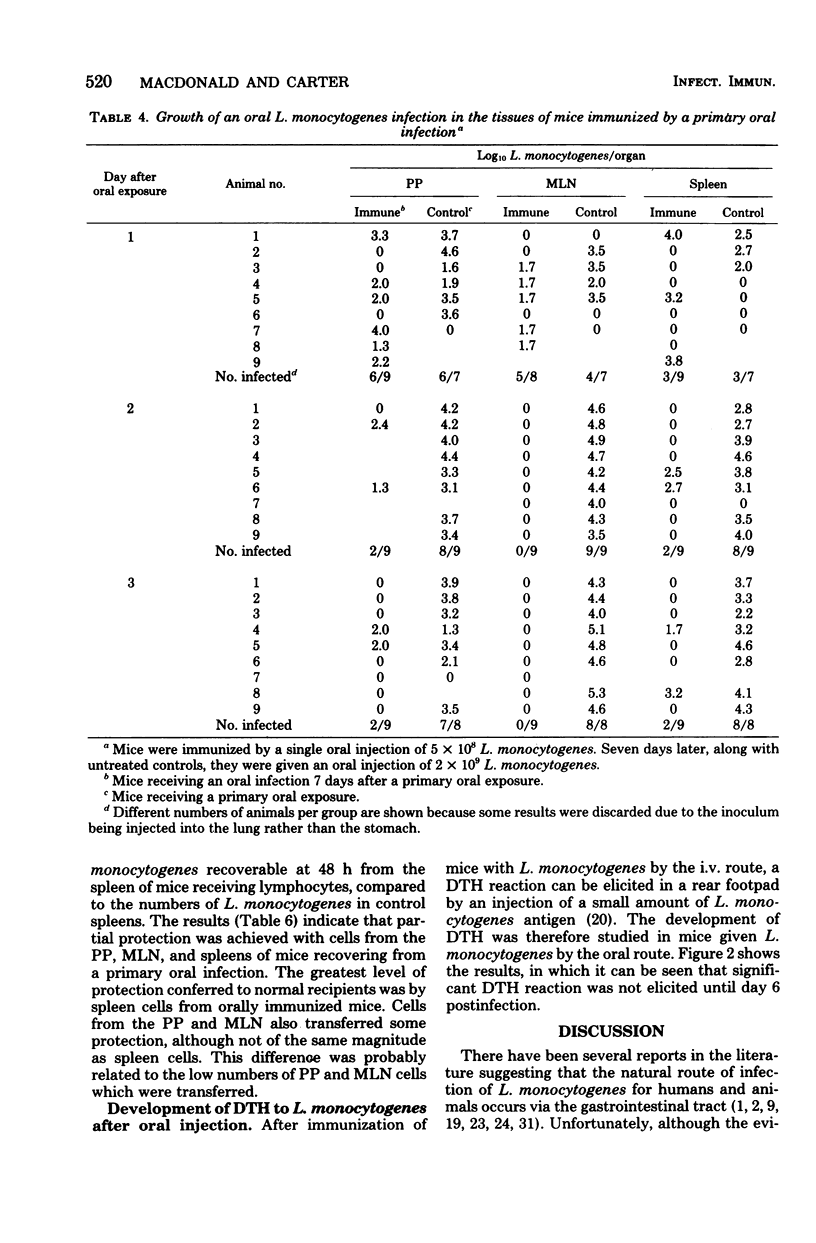
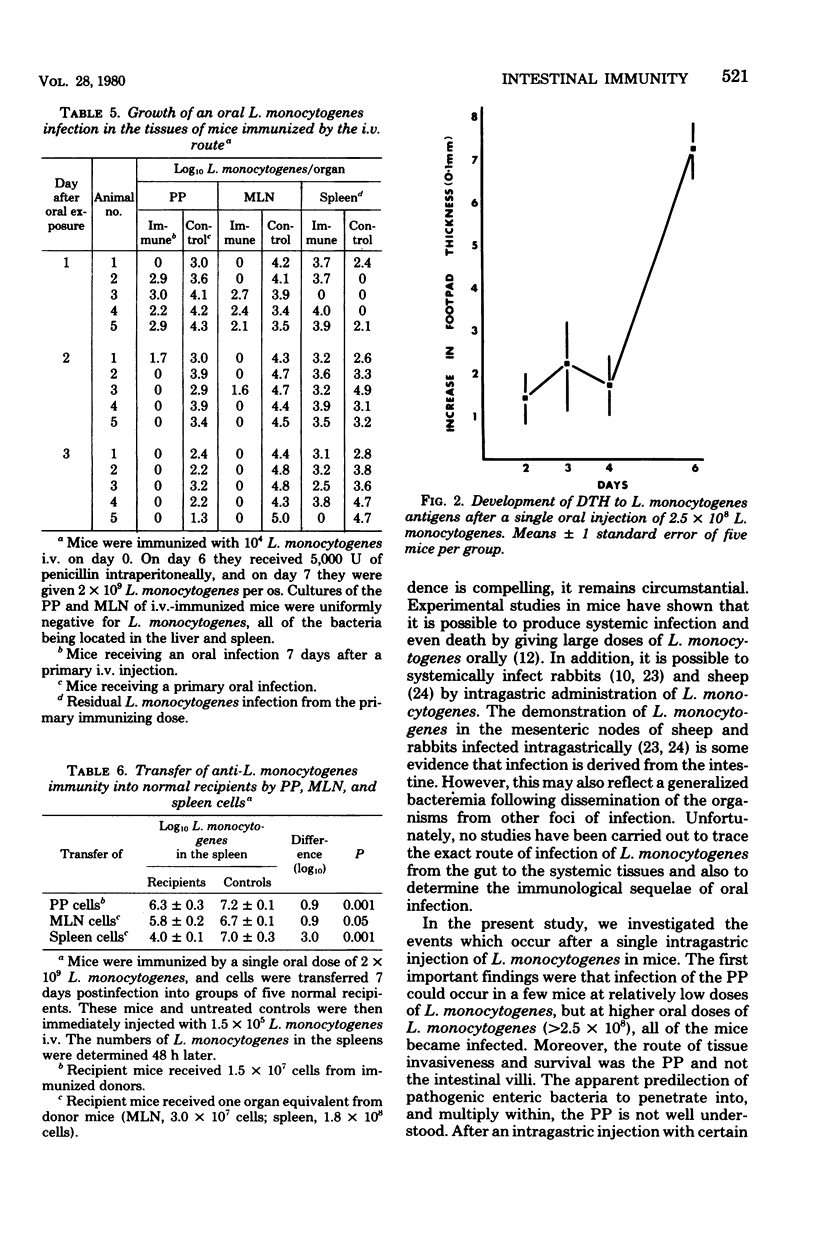
Specified pathogen-free B6D2F1 mice were orally infected with various doses of Listeria monocytogenes. Oral inocula containing more than 2.5 X 10(8) live organisms consistently initiated infection in the Peyer's patches (PP) of the small intestine. At lower doses the infection was sporadic, with many mice showing no apparent infection in the PP. The PP appeared to be the only site of tissue invasion and L. monocytogenes survival in the intestinal tissues, as no organisms were recovered from mucosa dissected free of all visible PP. Within the PP, the bacteria multiplied and the infection then disseminated to the mesenteric lymph node (MLN), liver, and spleen. However, bacteria were almost completely eliminated from all tissues, both systemic and gut-associated by 6 days postinfection. Mice given a primary L. monocytogenes infection by the oral route were highly resistant to subsequent intravenous or oral challenge. Likewise, sublethal intravenous infection rendered mice highly resistant to subsequent oral infection. In addition, lymphocytes from the PP, MLN, and spleens of mice recovering from a primary oral infection were able to adoptively transfer immunity to normal recipients. Finally, after oral infection, mice did not display peripheral delayed hypersensitivity to L. monocytogenes antigens until the organisms had penetrated to the spleen.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burn C. G. Clinical and Pathological Features of an Infection Caused by a New Pathogen of the Genus Listerella. Am J Pathol. 1936 May;12(3):341–348.1. [PMC free article] [PubMed] [Google Scholar]
- CORDY D. R., OSEBOLD J. W. The neuropathogenesis of listeria encephalomyelitis in sheep and mice. J Infect Dis. 1959 Mar-Apr;104(2):164–173. doi: 10.1093/infdis/104.2.164. [DOI] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. Experimental Yersinia enterocolitica infection in mice: kinetics of growth. Infect Immun. 1974 May;9(5):851–857. doi: 10.1128/iai.9.5.851-857.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. The route of enteric infection in normal mice. J Exp Med. 1974 May 1;139(5):1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B. Pathogenecity of Yersinia enterocolitica for mice. Infect Immun. 1975 Jan;11(1):164–170. doi: 10.1128/iai.11.1.164-170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Woolcock J. B., Collins F. M. Involvement of the upper respiratory tract in orally induced salmonellosis in mice. J Infect Dis. 1975 May;131(5):570–574. doi: 10.1093/infdis/131.5.570. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Carter P. B. Cellular immunity in enteric disease. Am J Clin Nutr. 1974 Dec;27(12):1424–1433. doi: 10.1093/ajcn/27.12.1424. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974 Dec;38(4):371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk W. P., McCormick J. N., Goodman J. R., Yoffey J. M., Fudenberg H. H. Peyer's patches: morphologic studies. Cell Immunol. 1970 Nov;1(5):500–520. doi: 10.1016/0008-8749(70)90038-9. [DOI] [PubMed] [Google Scholar]
- GRAY M. L., SINGH C., THORP F., Jr Abortion, stillbirth, early death of young in rabbits by listeria monocytogenes. II. Oral exposure. Proc Soc Exp Biol Med. 1955 May;89(1):169–175. doi: 10.3181/00379727-89-21747. [DOI] [PubMed] [Google Scholar]
- Hohmann A. W., Schmidt G., Rowley D. Intestinal colonization and virulence of Salmonella in mice. Infect Immun. 1978 Dec;22(3):763–770. doi: 10.1128/iai.22.3.763-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julianelle L. A. Biological and Immunological Studies of Listerella. J Bacteriol. 1941 Sep;42(3):367–383. doi: 10.1128/jb.42.3.367-383.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M. F., Campbell S. Functional characteristics of Peyer's patch lymphoid cells. I. Induction of humoral antibody and cell-mediated allograft reactions. J Exp Med. 1974 Feb 1;139(2):398–406. doi: 10.1084/jem.139.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Simon M. M., Hahn H. Specific Lyt 123 cells are involved in protection against Listeria monocytogenes and in delayed-type hypersensitivity to listerial antigens. J Exp Med. 1979 Oct 1;150(4):1033–1038. doi: 10.1084/jem.150.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFevre M. E., Vanderhoff J. W., Laissue J. A., Joel D. D. Accumulation of 2-micron latex particles in mouse Peyer's patches during chronic latex feeding. Experientia. 1978 Jan 15;34(1):120–122. doi: 10.1007/BF01921939. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Deissler J. F. Nature of "memory" in T-cell mediated antibacterial immunity: cellular parameters that distinguish between the active immune response and a state of "memory". Infect Immun. 1975 Oct;12(4):761–767. doi: 10.1128/iai.12.4.761-767.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Mackaness G. B. Immunological control of macrophage proliferation in vivo. Infect Immun. 1973 Jul;8(1):68–73. doi: 10.1128/iai.8.1.68-73.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSEBOLD J. W., INOUYE T. Pathogenesis of Listeria monocytogenes infections in natural host. I. Rabbit studies. J Infect Dis. 1954 Jul-Aug;95(1):52–66. doi: 10.1093/infdis/95.1.52. [DOI] [PubMed] [Google Scholar]
- OSEBOLD J. W., INOUYE T. Pathogenesis of Listeria monocytogenes infections in natural hosts. II. Sheep studies. J Infect Dis. 1954 Jul-Aug;95(1):67–78. doi: 10.1093/infdis/95.1.67. [DOI] [PubMed] [Google Scholar]
- Osebold J. W., Pearson L. D., Medin N. I. Relationship of antimicrobial cellular immunity to delayed hypersensitivity in Listeriosis. Infect Immun. 1974 Feb;9(2):354–362. doi: 10.1128/iai.9.2.354-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. L., Jones A. L. Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974 Feb;66(2):189–203. [PubMed] [Google Scholar]
- Sawicki W., Kucharczyk K., Szymanska K., Kujawa M. Lamina propria macrophages of intestine of the guinea pig. Possible role in phagocytosis of migrating cells. Gastroenterology. 1977 Dec;73(6):1340–1344. [PubMed] [Google Scholar]
- Seeliger H. P., Winkhaus-Schindl I., Andries L., Viebahn A. Die Isolierung von Listeria monocytogenes aus Stuhl-, Klärschlamm- und Erdproben. Pathol Microbiol (Basel) 1965;28(4):590–601. [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967 Jan;50(1):109–136. [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Blumershine R. V., Savage D. C. Association of Salmonella typhimurium with, and its invasion of, the ileal mucosa in mice. Infect Immun. 1975 Feb;11(2):365–370. doi: 10.1128/iai.11.2.365-370.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull P. C., Richmond J. E. A model of salmonella enteritis: the behaviour of Salmonella enteritidis in chick intestine studies by light and electron microscopy. Br J Exp Pathol. 1978 Feb;59(1):64–75. [PMC free article] [PubMed] [Google Scholar]
- Zachar Z., Savage D. C. Microbial interference and colonization of the murine gastrointestinal tract by Listeria monocytogenes. Infect Immun. 1979 Jan;23(1):168–174. doi: 10.1128/iai.23.1.168-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


