Abstract
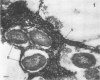
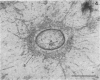
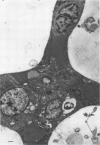
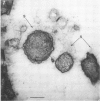
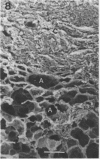
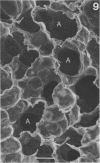
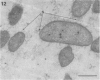
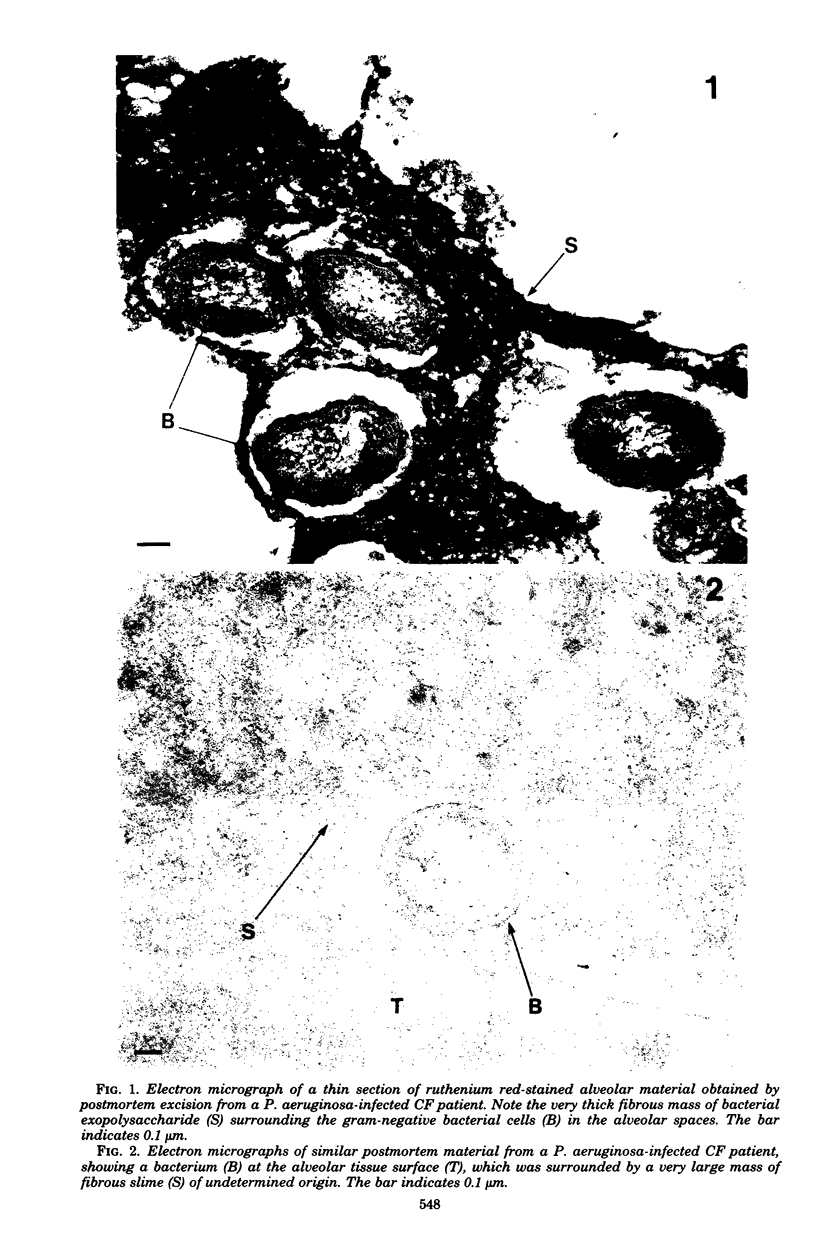
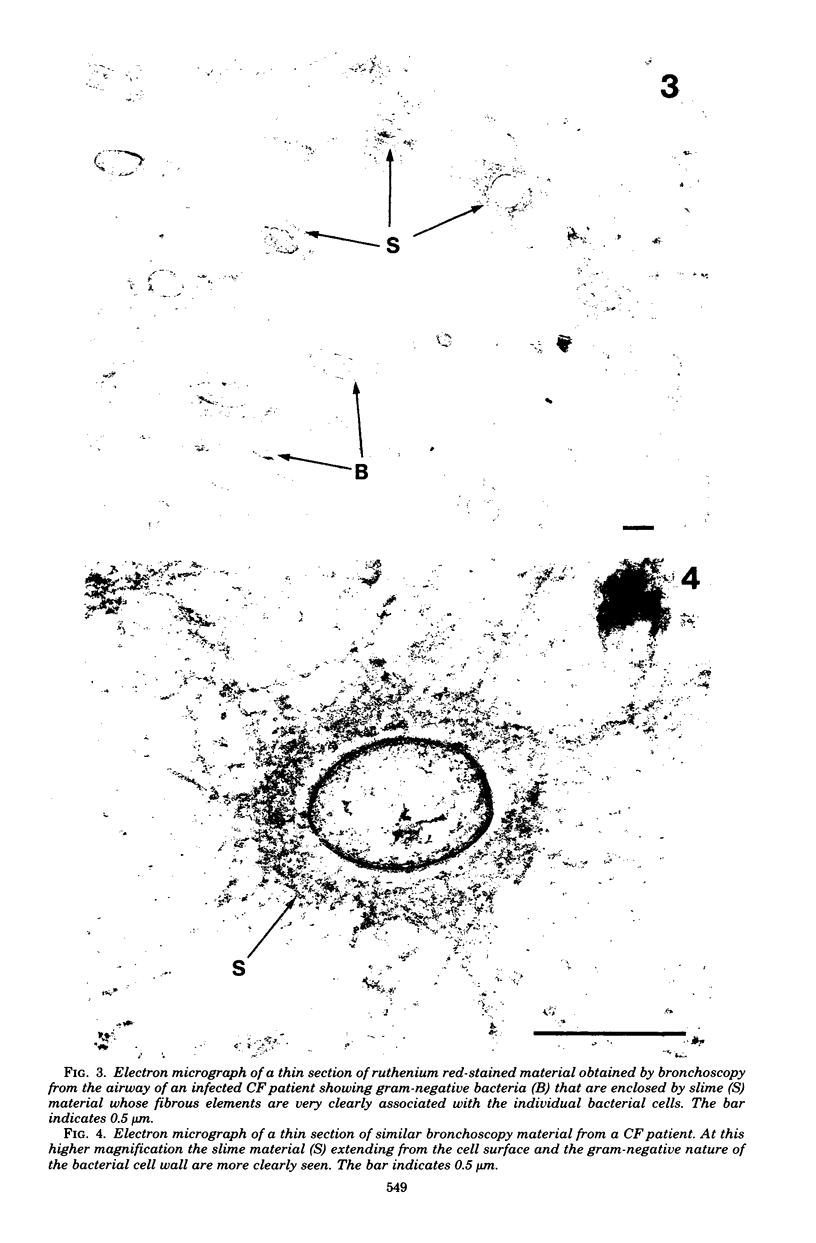
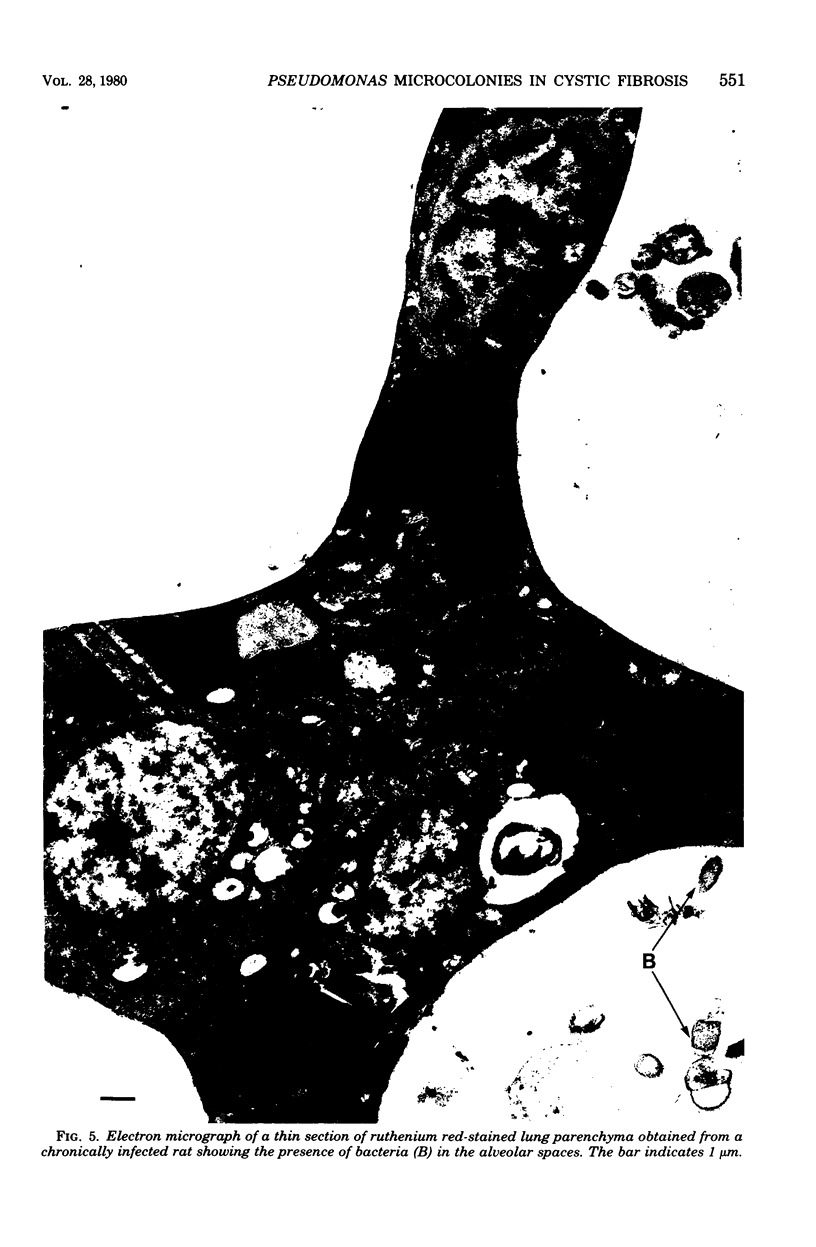
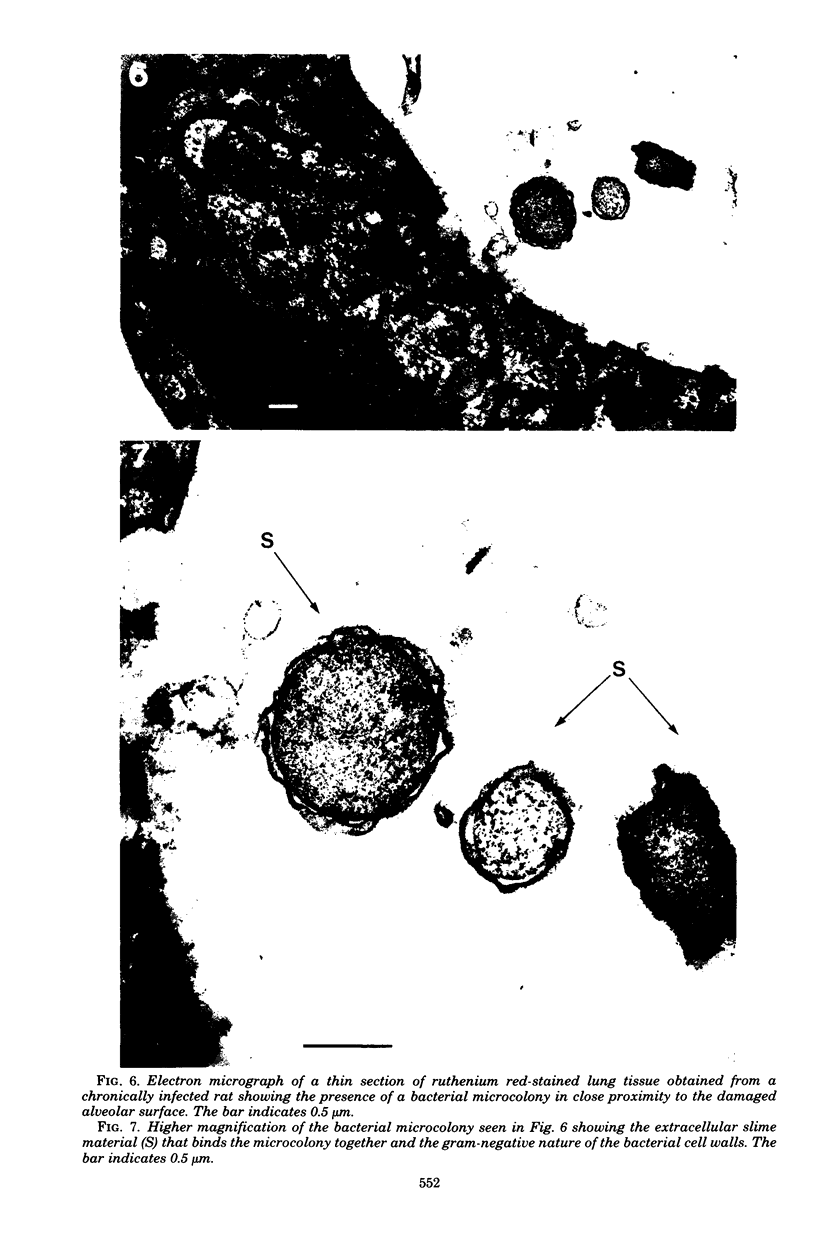
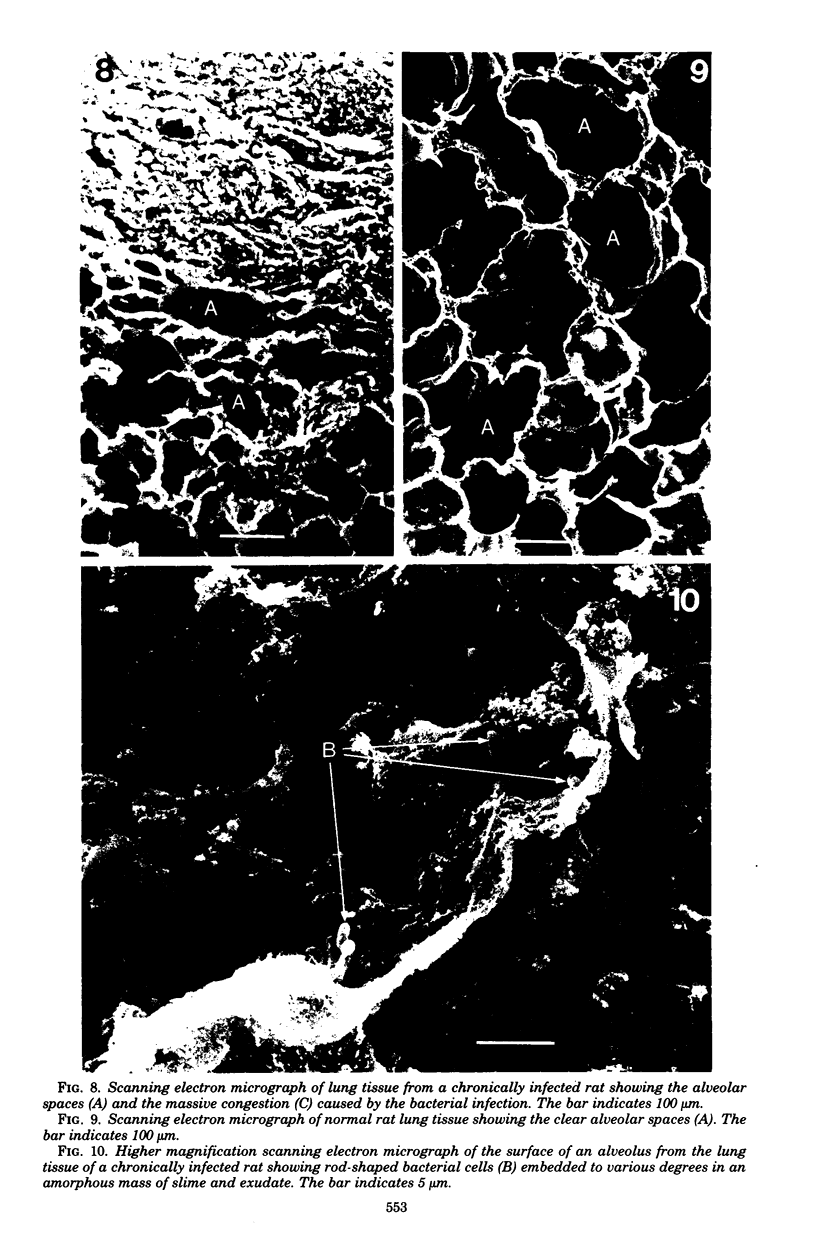
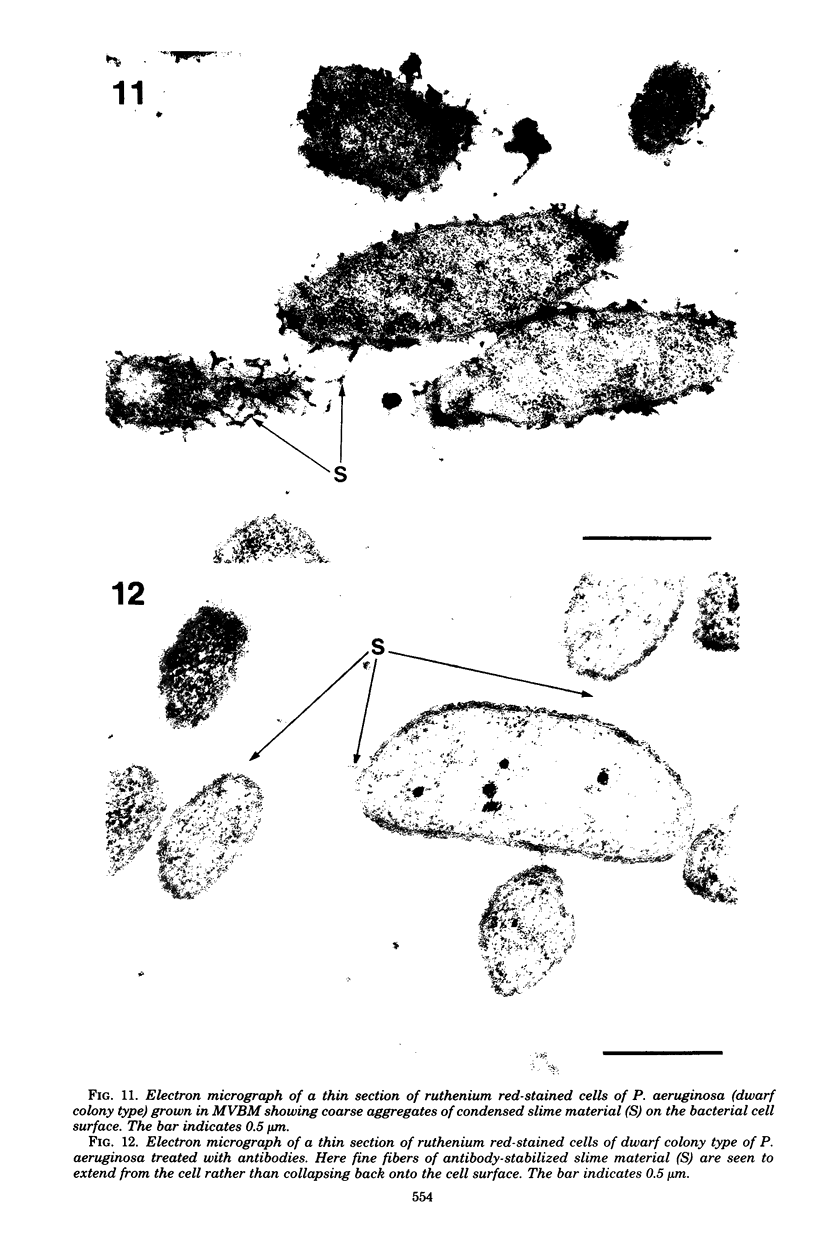
Direct electron microscopic examination of postmortem lung material from cystic fibrosis patients infected with Pseudomonas aeruginosa has shown that these bacterial cells form distinct fiber-enclosed microcolonies in the infected alveoli. Similar examination of bronchoscopy material from infected cystic fibrosis patients showed that the fibres of the enveloping matrix are definitely associated with the bacterial cells. The fibers of the extracellular matrix stain with ruthenium red and are therefore presumed to be polyanionic. When mucoid strains of P. aeruginosa were recovered from cystic fibrosis patients and grown in a suitable liquid medium, they were found to produce large microcolonies whose component cells were embedded in a very extensive matrix of polyanionic fibers that could be stabilized by reaction with antibodies to prevent collapse during the dehydration steps of preparation for electron microscopy. When these mucoid strains of P. aeruginosa were used to produce pulmonary infections of rats by the agar bead method, the infected alveoli contained large fiber-enclosed bacterial microcolonies. We conclude that the cells of P. aeruginosa that infect cystic fibrosis patients form microcolonies that are enveloped in a fibrous anionic matrix and that these microcolonies can be duplicated in in vitro cultures and in animal model systems.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977 May;130(2):911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash H. A., Woods D. E., McCullough B., Johanson W. G., Jr, Bass J. A. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am Rev Respir Dis. 1979 Mar;119(3):453–459. doi: 10.1164/arrd.1979.119.3.453. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Geesey G. G., Cheng K. J. How bacteria stick. Sci Am. 1978 Jan;238(1):86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOGGETT R. G., HARRISON G. M., WALLIS E. S. COMPARISON OF SOME PROPERTIES OF PSEUDOMONAS AERUGINOSA ISOLATED FROM INFECTIONS IN PERSONS WITH AND WITHOUT CYSTIC FIBROSIS. J Bacteriol. 1964 Feb;87:427–431. doi: 10.1128/jb.87.2.427-431.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett R. G., Harrison G. M., Carter R. E. Mucoid Pseudomonas aeruginosa in patients with chronic illnesses. Lancet. 1971 Jan 30;1(7692):236–237. doi: 10.1016/s0140-6736(71)90973-1. [DOI] [PubMed] [Google Scholar]
- Govan J. R. Mucoid strains of Pseudomonas aeruginosa: the influence of culture medium on the stability of mucus production. J Med Microbiol. 1975 Nov;8(4):513–522. doi: 10.1099/00222615-8-4-513. [DOI] [PubMed] [Google Scholar]
- Kulczycki L. L., Murphy T. M., Bellanti J. A. Pseudomonas colonization in cystic fibrosis. A study of 160 patients. JAMA. 1978 Jul 7;240(1):30–34. [PubMed] [Google Scholar]
- Laraya-Cuasay L. R., Cundy K. R., Huang N. N. Pseudomonas carrier rates of patients with cystic fibrosis and of members of their families. J Pediatr. 1976 Jul;89(1):23–26. doi: 10.1016/s0022-3476(76)80920-1. [DOI] [PubMed] [Google Scholar]
- Mackie E. B., Brown K. N., Lam J., Costerton J. W. Morphological stabilization of capsules of group B streptococci, types Ia, Ib, II, and III, with specific antibody. J Bacteriol. 1979 May;138(2):609–617. doi: 10.1128/jb.138.2.609-617.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malick L. E., Wilson R. B. Modified thiocarbohydrazide procedure for scanning electron microscopy: routine use for normal, pathological, or experimental tissues. Stain Technol. 1975 Jul;50(4):265–269. doi: 10.3109/10520297509117069. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Harding G. K., Ronald A. R., Dikkema J., Lam J., Hoban S., Costerton J. W. Influence of mucoidy on antibody coating of Pseudomonas aeruginosa. J Infect Dis. 1979 Mar;139(3):357–361. doi: 10.1093/infdis/139.3.357. [DOI] [PubMed] [Google Scholar]
- McCowan R. P., Cheng K. J., Bailey C. B., Costerton J. W. Adhesion of bacteria to epithelial cell surfaces within the reticulo-rumen of cattle. Appl Environ Microbiol. 1978 Jan;35(1):149–155. doi: 10.1128/aem.35.1.149-155.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]