Abstract
Key Points
Lamprey are cyclostomes, a group of vertebrates that diverged from lines leading to jawed vertebrates (including mammals) in the late Cambrian, 500 million years ago. It may therefore be possible to infer properties of photoreceptors in early vertebrate progenitors by comparing lamprey to other vertebrates.
We show that lamprey rods and cones respond to light much like rods and cones in amphibians and mammals. They operate over a similar range of light intensities and adapt to backgrounds and bleaches nearly identically.
These correspondences are pervasive and detailed; they argue for the presence of rods and cones very early in the evolution of vertebrates with properties much like those of rods and cones in existing vertebrate species.
Abstract
The earliest vertebrates were agnathans – fish‐like organisms without jaws, which first appeared near the end of the Cambrian radiation. One group of agnathans became cyclostomes, which include lamprey and hagfish. Other agnathans gave rise to jawed vertebrates or gnathostomes, the group including all other existing vertebrate species. Because cyclostomes diverged from other vertebrates 500 million years ago, it may be possible to infer some of the properties of the retina of early vertebrate progenitors by comparing lamprey to other vertebrates. We have previously shown that rods and cones in lamprey respond to light much like photoreceptors in other vertebrates and have a similar sensitivity. We now show that these affinities are even closer. Both rods and cones adapt to background light and to bleaches in a manner almost identical to other vertebrate photoreceptors. The operating range in darkness is nearly the same in lamprey and in amphibian or mammalian rods and cones; moreover background light shifts response–intensity curves downward and to the right over a similar range of ambient intensities. Rods show increment saturation at about the same intensity as mammalian rods, and cones never saturate. Bleaches decrease sensitivity in part by loss of quantum catch and in part by opsin activation of transduction. These correspondences are so numerous and pervasive that they are unlikely to result from convergent evolution but argue instead that early vertebrate progenitors of both cyclostomes and mammals had photoreceptors much like our own.
Keywords: adaptation, evolution, lamprey, photoreceptor, rhodopsin, vision
Key Points
Lamprey are cyclostomes, a group of vertebrates that diverged from lines leading to jawed vertebrates (including mammals) in the late Cambrian, 500 million years ago. It may therefore be possible to infer properties of photoreceptors in early vertebrate progenitors by comparing lamprey to other vertebrates.
We show that lamprey rods and cones respond to light much like rods and cones in amphibians and mammals. They operate over a similar range of light intensities and adapt to backgrounds and bleaches nearly identically.
These correspondences are pervasive and detailed; they argue for the presence of rods and cones very early in the evolution of vertebrates with properties much like those of rods and cones in existing vertebrate species.
Abbreviations
- GCAP
guanylyl cyclase‐activating protein
- P*
cone pigment molecules bleached
- Rh*
rhodopsins bleached
Introduction
The earliest vertebrates diverged from chordates during the Cambrian radiation (Smith et al. 2001) and were agnathans – fish‐like organisms lacking a jaw. About 500 million years ago, these animals or their vertebrate progenitors gave rise to cyclostomes, the group that includes the only agnathan species to have survived to the present day – hagfish and lamprey (Kuraku & Kuratani, 2006). At about the same time, other lines of agnathans gave rise to the first gnathostomes or jawed vertebrates, from which fishes, amphibians, reptiles, birds and mammals were all later derived.
Because the line leading to lamprey diverged from the rest of the vertebrates so long ago, it may be possible to infer some of the properties of early vertebrate progenitors by comparing lamprey to other vertebrate species. The earliest vertebrates had paired lateral eyes (Aldridge et al. 1993; Sansom et al. 2001) like those in lamprey and in the oldest fossils of jawed vertebrates (Carter, 1967). The presence of an eye is not by itself very informative, because well‐developed eyes with a focusing lens have appeared by convergent evolution in many animal lines, including for example spiders and squid. More informative are the properties of the cells within the eye. Research during the last few decades has shown that vertebrate rods and cones are remarkably uniform in their biochemistry and physiology, from fish and amphibians to mammals. Moreover many of these features are also found in lamprey. Lamprey photoreceptors have visual pigments closely resembling those of other vertebrates (Zhang & Yokoyama, 1997; Pisani et al. 2006), transducin G proteins (Muradov et al. 2008; Lamb et al. 2016) and a phosphodiesterase 6 effector enzyme (Muradov et al. 2007; Lamb et al. 2016). The lamprey retinal pigment epithelium contains the same enzymes other vertebrates use to regenerate all‐trans to 11‐cis retinal (Poliakov et al. 2012). Moreover we have recently shown that the lamprey Petromyzon marinus has two kinds of photoreceptors – a rod and a single spectral class of cone, whose responses closely resemble those of rods and cones of jawed vertebrates (Morshedian & Fain, 2015). Lamprey rods respond to single photons with a sensitivity not very different from mouse and are about 75–100 times more sensitive than lamprey or mouse cones (see also Asteriti et al. 2015).
We now show that the correspondences between lamprey and other vertebrate photoreceptors are even more profound. Both rods and cones in lamprey adapt to background light with changes in sensitivity and response waveform much like those described for salamander or mouse. Lamprey rods and cones are also desensitized by strong bleaching light in a similar manner. These resemblances are so numerous and so close in detail that they are unlikely to be the result of convergent evolution. Instead they argue that, before the divergence of cyclostomes from gnathostomes 500 million years ago, primitive vertebrates had photoreceptors nearly like our own.
Methods
Ethical approval and animals
Experiments were performed in accordance with rules and regulations of the NIH guidelines for research animals, as approved by the institutional animal care and use committee of the University of California, Los Angeles, USA. Adult sea lamprey (Petromyzon marinus) were provided to us by the Hammond Bay Biological Station of the United States Geological Survey (USGS), Millersburg, MI, USA. They were captured in tributaries of Lake Huron (Ocqueoc River and Cheboygan River) in the process of their upstream spawning migration and shipped to our laboratory with a permit from the Department of Fish and Wildlife of the California Natural Resource Agency. Animals were kept for no more than 6 weeks in a large tank filled with constantly circulating de‐chlorinated water chilled to 4°C with an aquarium chiller (AquaEuroUSA, Gardena, CA, USA). The tank was kept in a cold room at 10°C and was maintained under cyclic 12 h on–12 h off room lighting. All animals were dark adapted prior to every experiment for at least 3 h. They were anaesthetized by immersion in 250 mg l−1 tricaine methane sulfonate, decapitated, and pithed rostrally and caudally before removal of the eyes.
Tissue dissection and preparation
Eyes were enucleated under dim red light. The anterior portion of the eye was cut and the lens and cornea were removed with infrared image converters. The retina was isolated from the eyecup; the retinal pigment epithelium was removed with fine tweezers, and the retina was chopped into small pieces with a razor blade. The retinas were exposed for 3 min to 0.5 mg ml−1 collagenase and 0.33 mg ml−1 hyaluronidase to prevent clogging of pipettes by vitreous humour and extracellular matrix. The pieces were then transferred to the recording chamber in complete darkness by means of infrared goggles (ATN Corporation, South San Francisco, CA, USA). To record from cones, we selected cells previously referred to as ‘long’ photoreceptors (see for example Dickson & Graves, 1979; Govardovskii & Lychakov, 1984), whose outer segments extend further away from the cells of the inner retina in the direction of the choroid. The responses of the shorter rods were recorded by moving the electrode between cones or by searching for parts of the slice where cones had been displaced during preparation.
Solutions and pipettes
During recording the photoreceptors were continuously perfused with the same solution used for the dissection, which contained (in mm) 93 NaCl, 2.1 KCl, 2.6 CaCl2,, 1.8 MgCl2, 2.0 NaHCO3, 10.8 Hepes and 4.0 glucose. The solution was bubbled with 5% CO2–95% O2 at pH 7.4. The recording electrodes were filled with Locke's solution, which contained (in mm) 93 NaCl, 2.1 KCl, 2.6 CaCl2, 1.8 MgCl2, 2.0 NaHCO3 and 10.8 Hepes at pH 7.4. Fire‐polished borosilicate glass was pulled with a micropipette puller (P‐97, Sutter Instruemnt Co., Novato, CA, USA) to produce pipettes with rapidly tapering shanks. The tip size was further adjusted under a compound microscope by moving the pipette close to a platinum heating wire until the tip had melted to an inner diameter that would fit the outer segment of the photoreceptor and provide a good seal. The resistance of useable pipettes when filled with solution was about 3–4 MΩ for rods and 2.5–3 MΩ for cones.
Suction‐electrode recording
Responses of single photoreceptor outer segments were recorded at room temperature with the suction‐electrode technique (Baylor et al. 1979) as in previous experiments (Morshedian & Fain, 2015). The change in outer‐segment membrane current produced by a stimulus was recorded with a current‐to‐voltage converter (Axopatch 200A; Molecular Devices, Sunnyvale, CA, USA), low‐pass filtered at 30 Hz with an 8‐pole Bessel filter (Kemo Limited, Dartford, Kent, UK), and sampled at 100 Hz. Digitized data were recorded with Clampex 8.0 and were analysed with Origin Pro (OriginLab Inc, Northampton, MA, USA). Curve fitting and plotting of data were also done in Origin.
Cells were stimulated with a dual‐beam optical bench; the light of halogen lamp bulbs was passed through electronic shutters (Uniblitz, Vincent Associates, Rochester, NY, USA) and interference filters at wavelengths of 500 nm for rods and 600 nm for cones, near the peaks of spectral sensitivities of both receptors (Morshedian et al. 2017). The intensity of the light was attenuated with absorptive neutral‐density filters. The light intensity was calibrated with a photodiode (UDT Instruments, San Diego, CA, USA, formerly Graseby Optronics). Because there is some uncertainty about photoreceptor dimensions and the specific absorbance of lamprey photoreceptor pigments (Hárosi & Kleinschmidt, 1993; Morshedian et al. 2017), we calculated intensities in units of rhodopsins bleached (Rh*) from the dark‐adapted response–intensity curve (Fig. 3 A) and the amplitude of the single‐photon response of lamprey rods (Morshedian & Fain, 2015). For cones, we estimated intensities in units of cone pigment bleaching (P*) from intensities in Rh* for rods and the ratio of the volume of the cone to rod outer segment (about 0.4, see Dickson & Graves, 1979; Hárosi & Kleinschmidt, 1993).
Figure 3. Background light decreases rod sensitivity and accelerates response decay.

A, mean peak response amplitude (± SEM) as a function of flash intensity for the cells of Fig. 2; colours indicate background intensity (in Rh* s−1) as follows: black, 0 (no background); red, 12; green, 32; blue, 122; orange, 330; and purple, 1190. Data for each background have been fitted with eqn (2) with the following values of r max (in pA) and a (in Rh* −1), from dimmest to brightest background: 13.1, 0.038; 11.6, 0.034; 10.6, 0.029; 8.8, 0.017; 5.8, 0.0083; 3.6, 0.0051. B, superimposed responses from Fig. 2 to the same flash of 31 Rh* in each of the background illuminations; same colour coding as for A. Note monotonic decrease in response amplitude with increasing background intensity. C, superimposed responses from B normalized cell by cell to the same peak amplitude to illustrate acceleration of rate of response decay with increasing background illumination. Best‐fitting single exponential decay time constants (τrec) averaged cell by cell were as follows, from dark to background of 1190 Rh* s−1 in ms: 922 ± 18, 925 ± 20, 765 ± 9, 564 ± 12, 435 ± 10 and 364 ± 16. [Color figure can be viewed at wileyonlinelibrary.com]
Bleaching and pigment regeneration
Pigment bleaching could not be done on the apparatus we used for recording because the light bench was not sufficiently bright. We therefore bleached photoreceptors after isolation and before recording with a separate apparatus, in which the photoreceptors were illuminated directly. The fraction of pigment bleached is independent of the photoreceptor collecting area and could be estimated from:
| (1) |
where F is the fraction bleached, I is the intensity of the bleaching light, t is the time of exposure of the bleaching light, and P is the in situ photosensitivity of vertebrate photopigment (5.7 × 10−9 μm2, see Woodruff et al. 2004; Nymark et al. 2012). The use of this equation assumes that the photosensitivity of lamprey visual pigment is the same as that in other vertebrates, and that there was no pigment regeneration. Because the build‐up of retinoids inside the outer segments of photoreceptors after bleaching may be deleterious, pigments were bleached at a slow rate, and 0.1% bovine serum albumin (BSA) was added to the solution bathing the photoreceptors during bleaching to facilitate removal of free retinoid from the cells (Nymark et al. 2012). The cells were left in BSA solution for 1 h after the bleach, and the solution containing the BSA was then replaced with the normal perfusion medium before recording. For the experiments of Fig. 9, we obtained 11‐cis retinal as a gift from the laboratory of Rosalie Crouch at the Medical University of South Carolina. The 11‐cis retinal was dissolved in an aqueous/ethanol solution for delivery to the photoreceptors as in Frederiksen et al. (2012). The peak absorbance of 11‐cis retinal was measured with a spectrophotometer (Shimadzu UV‐2101 PC) and adjusted to a final concentration of 30 μm, which was applied for 45–90 min to the photoreceptors.
Figure 9. Recovery from bleach after exposure to exogenous 11‐cis retinal.
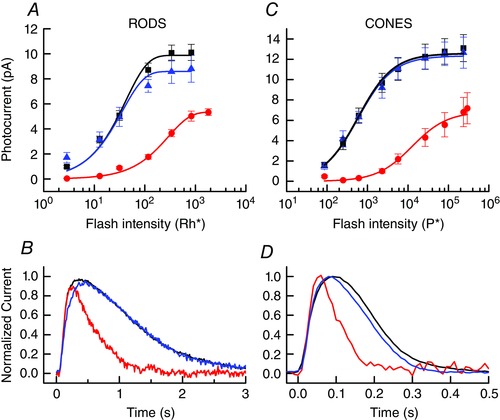
A, mean peak response amplitude (± SEM) as a function of flash intensity for 8–13 rods in darkness before bleach (black), at steady state after 50% bleach (red), and at steady state after exposure to 11‐cis retinal (blue, see Methods). Data have been fitted with eqn (2) with the following values of r max (in pA) and a (in Rh* −1): 9.9, 0.024; 5.4, 0.0034; 8.6, 0.028. B, superimposed responses to 20 ms flashes of 117 Rh* from rods of A, normalized to same peak amplitude to illustrate change in rate of response decay after bleaching and pigment regeneration. Same colour coding as in A. C, mean peak response amplitude (± SEM) as a function of flash intensity for 6–10 cones in darkness before bleach (black), at steady state after 90% bleach (red), and at steady state after exposure to 11‐cis retinal (blue). Data have been fitted with eqn (3) with the following values of r max (in pA) and I ½ (in P*): 12.6, 584; 6.9, 12600; 12.3, 579. D, superimposed responses to 20 ms flashes of 5640 P* from cones of C, normalized to the same peak amplitude to illustrate change in rate of response decay after bleaching and pigment regeneration. Same colour coding as in C. [Color figure can be viewed at wileyonlinelibrary.com]
Statistical analysis
The results are expressed as means ± standard error of the mean (SEM). Continuous variables were compared by a two‐tailed Student's t test.
Results
Part one: background adaptation
Rods and cones in amphibians and mammals adapt to steady background light with an initial change in current that relaxes with time. At steady state, photoreceptor sensitivity decreases with increasing background intensity to preserve a constant threshold to contrast (see Fain et al. 2001; Arshavsky & Burns, 2012; Morshedian & Fain, 2014). Rods are more sensitive and their responses to brief stimuli decay more slowly in order to integrate photons at dim intensities and achieve high sensitivity; they adapt over a limited range and then saturate in brighter light. Cones are less sensitive but decay more rapidly to respond more accurately to change and motion when photons are no longer limiting. Cones never saturate and continue to function even in bright light. In this part of the Results, we show that background adaptation also occurs in lamprey rods and cones in a manner nearly identical to that of rods and cones of jawed vertebrates. In part 2 of the Results, we describe adaptation after exposure to bleaching illumination, and recovery of sensitivity after pigment regeneration.
Responses to light steps
One of the characteristic features of adaptation in any sensory receptor is a time‐dependent decrease in the response to maintained stimulation (Fain, 2003). Previous experiments on amphibians and mammals have shown that when a rod or cone is exposed to a maintained step of light, the response rises to a peak and then partially recovers as the photoreceptor adapts to the light (Matthews et al. 1990; Calvert et al. 2002; Chen et al. 2010b; Sakurai et al. 2015). Figure 1 A and B illustrates a similar phenomenon for lamprey rods and cones. As in earlier experiments on amphibian and mouse rods (for example Calvert et al. 2002; Chen et al. 2010b), the time course of channel reopening consists of an initial relaxation of the response (Fig. 1 A and B, insets), which at the light intensities used for these experiments had time constants of 3.3 s for rods and 850 ms for cones. This fast phase was then followed by a slow continuing decrease in photocurrent, which in Fig. 1 had time constants of 36 s for rods and 7.8 s for cones. Both phases of recovery were significantly faster in cones than in rods (Student's t test, P < 10−6). The turning off of light produced a small transient overshoot in cones, during which the current became larger than its resting value in darkness. This phenomenon was smaller or absent in lamprey rods.
Figure 1. Change in circulating current to steps of light.
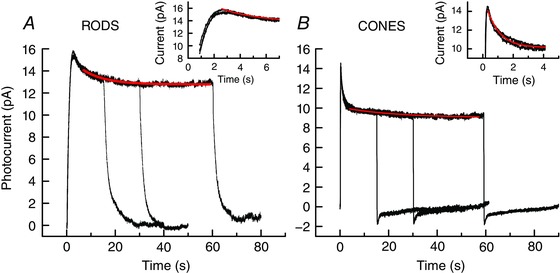
A, lamprey rods. Superimposed means of 7 rods each exposed 3 times to 15, 30 and 60 s steps at intensity of 117 Rh* s−1 at 500 nm. Red trace is best‐fitting single‐exponential decay with time constant of 36 s. Inset: first 7 s at a higher time resolution; red trace is best‐fitting single‐exponential decay with time constant of 3.3 s. B, lamprey cones. Superimposed means of 8 cones each exposed 3 times to 15, 30 and 60 s steps at intensity of 4030 P* s−1 at 600 nm. Red trace is best‐fitting single‐exponential decay with time constant of 7.8 s. Inset: first 4 s at a higher time resolution; red trace is best‐fitting single‐exponential decay with time constant of 850 ms. [Color figure can be viewed at wileyonlinelibrary.com]
Responses to light increments: rods
A second nearly universal characteristic of sensory adaptation is a decrease in the sensitivity of the receptor to stimulus increments superimposed on top of maintained stimulation. For photoreceptors, this decrease in sensitivity is accompanied by a characteristic acceleration of the time course of decay, which is part of a mechanism to increase the ability of the visual system to detect change and motion in increasing ambient illumination (see Fain et al. 2001). To explore the nature of adaptation in lamprey photoreceptors, we therefore presented maintained illumination as in Fig. 1 and then gave brief flashes on top of the continuous light to assess changes in response sensitivity and waveform.
In Fig. 2, we show responses of lamprey rods to a series of increasing flash intensities in darkness and in the presence of five background illuminations of increasing intensity. These experiments were performed in the following way. Responses were first recorded with no background light (Fig. 2 A). Then the first background was presented and maintained for 2 min, which we found to be sufficient for the photocurrent level and sensitivity to reach steady state. After responses to flashes had been recorded, the background was extinguished, and we waited 3–5 min to allow the cell to recover before presenting the next background. Both flashes and backgrounds proceeded from dim to bright, and each trace is the mean of five flash presentations for each of eight rods.
Figure 2. Background adaptation of lamprey rods.
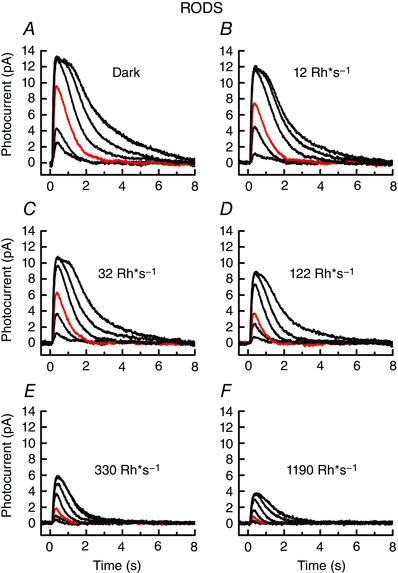
Responses to 20 ms flashes at 500 nm, 2 min after exposure to background light. Data are means of 5 flashes from each of 8 cells; for each cell the entire series was recorded. A–D, flash intensities were (in Rh*) 3, 13, 31, 117, 338 and 829; and E–F, 3, 13, 31, 117, 338, 829 and 3150. Red traces are responses to 31 Rh*. Background intensities were as follows (in Rh* s−1): A, dark‐adapted (no background); B, 12; C, 32; D, 122; E, 330; and F, 1190. [Color figure can be viewed at wileyonlinelibrary.com]
As the background light was increased, the peak amplitude of the response to flashes decreased (Fig. 2 B–F), reflecting the decrease in the circulating current of the rod produced by the maintained response to the background. The background also produced a substantial decrease in sensitivity, as can be clearly seen from the amplitude of the red traces in each flash series, which are the responses of the rods at each background to the same flash intensity of 31 Rh*.
A more complete description of the effect of the background on flash response amplitude can be obtained from the response–intensity curves plotted for these cells in Fig. 3 A. The increase in background light produced both a decrease in maximum amplitude and a shifting of the curves along the intensity axis, reflecting the decrease in sensitivity produced by the background. The curves in Fig. 3 A have been fitted with the following equation (Lamb et al. 1981):
| (2) |
where r is the peak response amplitude, r max is the maximum value of r in bright light, I is the flash intensity in Rh*, and a is a constant with units of Rh* −1. The change in the value of a reflects the change in sensitivity, because the smaller the value of a, the greater must be the flash intensity I required to produce a response of the same relative amplitude r/r max. The fits in Fig. 3 A show that both r max and a progressively decrease as the response–intensity curves shift downward and to the right with increasing background intensity.
Background light also produced an acceleration of the decay phase of the flash response. In Fig. 3 B we have superimposed the red responses in Fig. 2 to the same flash intensity of 31 Rh*, and we then normalized each of the responses cell by cell to the same peak amplitude in Fig. 3 C. The declining phase of the response could be adequately described with a single‐exponential decay function, whose time constant τrec monotonically decreased from 932 ± 18 ms for the dark‐adapted response to 364 ± 16 ms in the brightest background (see legend to Fig. 3).
Responses to light increments: cones
In Figs 4 and 5, we give similar results for lamprey cones, which show a comparable decrease in sensitivity and acceleration of response decay. There were, however, some important differences. Cone responses were considerably less sensitive, and their the response–intensity curves in Fig. 5 A were poorly fit by eqn (3), an equation derived for a model of transduction which may not be applicable to cones (Lamb et al. 1981). We found instead that these curves could be adequately described by a Michaelis–Menten equation of the form:
| (3) |
where r is the peak response amplitude, r max is the maximum value of r in bright light, I is the flash intensity in P*, and I ½ is a constant (in units of P*) equal to the flash intensity producing a half‐maximal response. The best fitting value of I ½ for dark‐adapted lamprey cones was 574 P*, increasing to 9700 P* in the brightest background.
Figure 4. Background adaptation of lamprey cones.
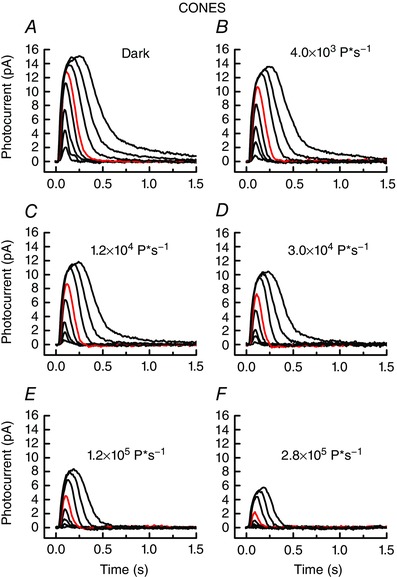
Responses to 20 ms flashes at 600 nm, 2 min after exposure to background light. Data are means of 5 flashes from each of 11 cells; for each cell the entire series was recorded. Flash intensities were (in P*) 85, 246, 604, 2300, 5640, 26,500, 80,800 and 235,000. Red traces are responses to 5640 P*. Background intensities were as follows (in P* s−1): A, dark‐adapted (no background); B, 4030; C, 12,300; D, 30,200; E, 115,000; and F, 282,000. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 5. Background light decreases cone sensitivity and accelerates response decay.
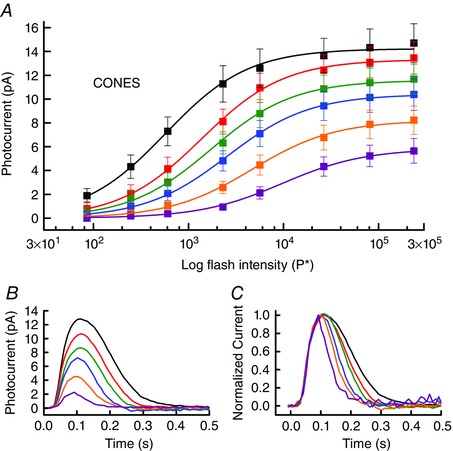
A, mean peak response amplitude (± SEM) as a function of flash intensity for the cells of Fig. 4; colours indicate background intensity (in P* s−1) as follows: black, 0 (no background); red, 4030; green, 12,300; blue, 30,200; orange, 115,000; and purple, 282,000. Data for each background have been fitted with eqn (3) with the following values of r max (in pA) and I ½ (in P* −1), given from dimmest to brightest background: 14.2, 574; 13.3, 1370; 11.6, 1730; 10.4, 2560; 8.2, 4680; 5.8, 9700. B, superimposed responses from Fig. 4 to same flash of 5640 P* in each of the background illuminations; same colour coding as for A. Note monotonic decrease in response amplitude with increasing background intensity. C, superimposed responses from B normalized to the same peak amplitude to illustrate monotonic acceleration of rate of response decay with increasing background illumination. Best‐fitting single exponential decay time constants (τrec) averaged cell by cell were as follows, from dark to background of 282,000 P* s−1 in ms: 181 ± 27, 129 ± 17, 99 ± 10, 70 ± 6, 56 ± 3 and 42 ± 2. [Color figure can be viewed at wileyonlinelibrary.com]
Another difference between lamprey rods and cones is the much faster rate of decay of the cone response. Even in the brightest background, the mean value of τrec for rods was 364 ms, whereas the mean value of τrec for dark‐adapted cones was only half as great at 181 ms, decreasing to 42 ms in the brightest background (see legend to Fig. 5).
Weber–Fechner relation for lamprey rods and cones
The sensitivity of rods and cones in amphibians and mammals decreases in background light according to the Weber–Fechner relation, which specifies that sensitivity changes nearly in inverse proportion to the intensity of the background to preserve a constant threshold to contrast (Fain, 1976; Matthews et al. 1988, 1990; Mendez et al. 2001; Chen et al. 2010b; Sakurai et al. 2011). In Fig. 6, we have plotted the sensitivity of lamprey rods and cones as a function of background intensity. Sensitivity was calculated from small‐amplitude responses (r < 0.3r max) for the cells of Figs 2, 3, 4, 5 in each of the background lights. The curves in Fig. 6 are the Weber–Fechner relation:
| (4) |
where S F is the flash sensitivity of the photoreceptor in the presence of a background, is the flash sensitivity in darkness, I B is the intensity of the background (in Rh* s−1 or P* s−1), and I 0 is a constant called the ‘dark light’, equal to the background intensity required to reduce sensitivity by one‐half. Best‐fitting values for I 0 were 67 Rh* s−1 for rods and 7070 P* s−1 for cones, which differ by a factor of over 100 reflecting the decreased sensitivity of the cone and its shorter integration time.
Figure 6. Weber–Fechner relations for lamprey rods and cones.
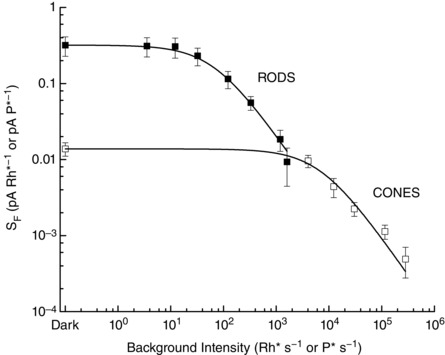
Backgrounds and flashes were at 500 nm for rods and 600 nm for cones; intensities are given separately in units of Rh* or Rh* s−1 for rods and P* or P* s−1 for cones. Sensitivity (S F) was calculated by dividing the peak amplitude of responses within the linear range (r < 0.3r max) by the flash intensity in Rh* or P*. Data have been fitted with eqn (4) with values of I 0 of 67 Rh* s−1 for rods and 7070 P* s−1 for cones.
At the brightest background for rods in Fig. 6 (1190 Rh* s−1), sensitivity was modestly below the value predicted by eqn (4). When the background intensity was then increased further even by a factor of only 3, no responses could be recorded to the brightest incremental flashes we gave, in contrast to an earlier report of Govardovskii & Lychakov (1984) from electroretinogram recording for the lamprey Lampetra fluviatilis. Incremental saturation of rod function was first observed for human vision (Aguilar & Stiles, 1954) but has since been documented for single rods, for example from toad (Fain, 1976) and mouse (Chen et al. 2010b). Fig. 6 shows that in lamprey, incremental saturation occurs at about the intensity at which cones begin to adapt. Further increases in background intensity leave rods unresponsive, but cones continue to operate with a sensitivity in good agreement with eqn (4).
At the brightest background intensities for cones, some of the decrease in sensitivity with increasing background intensity was produced by bleaching of the cone photopigment, which would have been significant. For the brightest background, we estimate that over 90% of the cone pigment would have been bleached in the time sensitivity was measured during exposure to the background. Bleaching may have influenced the position and shape of the response–intensity curve, because the bleaching was progressive, and little or no regeneration of pigment would have occurred in an isolated photoreceptor. Even with considerable bleaching of pigment, however, cone sensitivity continued to follow the Weber–Fechner relation, as previously observed for salamander and turtle cones (Matthews et al. 1990; Burkhardt, 1994). Moreover we never observed any indication of incremental saturation of the cone response.
Part 2: bleaching adaptation
When an isolated photoreceptor from an amphibian or mammal is exposed to light bright enough to bleach a significant fraction of the photopigment, and no retinal pigment epithelium and Müller cells are present to convert the bleached all‐trans retinal back to 11‐cis retinal, the photoreceptor is initially greatly desensitized, and rods are saturated and completely unable to respond (see Fain et al. 2001). With time in darkness, however, the photoreceptors recover to a steady state with stable values of response amplitude and sensitivity which are smaller than before the bleach. These changes in response properties are known to be produced in part from a decrease in the probability of photon absorption, due to a reduction in the concentration of unbleached pigment; and in part from light adaptation produced by activation of the visual cascade by opsin (see Fain et al. 2001). The photoreceptor can be almost entirely returned to its dark‐adapted condition if the 11‐cis chromophore is introduced to the cell exogenously (for example Berry et al. 2016). In the following section we show that bleaching adaptation also occurs in lamprey rods and cones and has properties much as in other vertebrates.
Responses of bleached lamprey rods and cones
In Fig. 7, we show responses to increasing flash intensities for lamprey rods (Fig. 7 A–C) and cones (Fig. 7 D–F) before bleaching and at steady state 1 h after bleaches of 50% and 90% of the photopigment. Bleaching altered the response properties of the cells much as did steady background illumination (Figs 2 and 4): peak response amplitude was smaller, and the sensitivity of the photoreceptor was decreased. The effect on sensitivity is apparent from the red traces, which are responses to the same flash intensity for rods of 117 Rh* (in Fig. 7 A–C), and for cones of 5640 P* (in Fig. 7 D–F).
Figure 7. Bleaching adaptation: mean responses to 20 ms flashes.
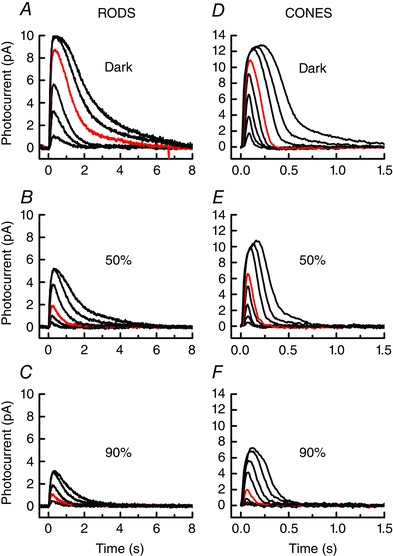
A, dark‐adapted rods (n = 11); flash intensities (in Rh*) were 3, 13, 31, 117, 338 and 829. B, rods at steady state after 50% bleach (n = 11); flash intensities were (in Rh*) 13, 31, 117, 338, 829 and 1810. C, rods at steady state after 90% bleach (n = 11); flash intensities (in Rh*) were 31, 117, 338, 829 and 1810. D, dark‐adapted cones (n = 13); flash intensities (in P*) were 85, 246, 604, 2300, 5640, 26,500, 80,800 and 235,000. E, cones at steady state after 50% bleach (n = 7); flash intensities as in E. F, cones at steady state after 90% bleach (n = 7); flash intensities were (in P*) 246, 604, 2300, 5640, 26,500, 80,800, and 235,000 and 293,000. Red traces are responses to 117 Rh* in A–C and 5640 P* in D–F. [Color figure can be viewed at wileyonlinelibrary.com]
In Fig. 8 A and C, we have plotted the peak amplitude of rod and cone responses from Fig. 7 as a function of flash intensity, including also responses to 10% bleaches. As in Figs 3 A and 5 A, we have fitted these data with eqn (2) for rods and eqn (3) for cones. Bleaching shifted the response–intensity curves downward and to the right, much as has been previously observed for other vertebrates (for example Nymark et al. 2012). The changes in sensitivity are consistently greater than would be predicted from the loss of quantum catch. A 50% bleach would reduce the probability of photon absorption by a factor of only 2, whereas both rods and cones in Fig. 8 A and C show decreases in sensitivity that are considerably greater.
Figure 8. Bleaching decreases sensitivity and accelerates response decay.
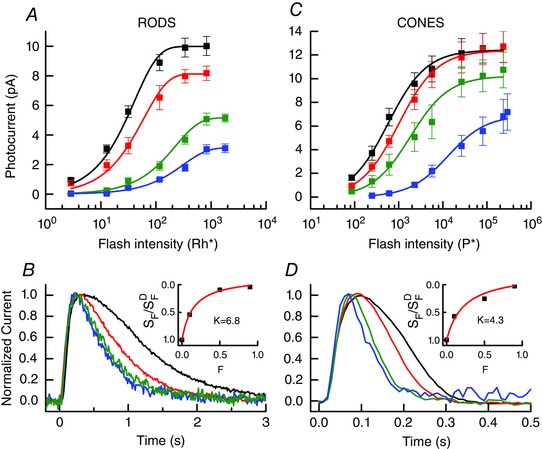
A, mean peak response amplitude (± SEM) as a function of flash intensity for the rods of Figs 7 A–C. Colours indicate bleaching condition as follows: black, dark‐adapted (no bleaching); red, 10% bleach; green, 50% bleach; blue, 90% bleach. Data have been fitted with eqn (2) with the following values of r max (in pA) and a (in Rh* −1), given in the order of dark to 90% bleach: 10.0, 0.024; 8.1, 0.016; 5.1, 0.0044; 3.1, 0.0032. B, superimposed responses to 20 ms flashes of 117 Rh* from rods of Figs 7 A–C and 8A, normalized to same peak amplitude to illustrate acceleration of rate of response decay after bleaching. Same colour coding as in A. Best‐fitting single exponential decay time constants (τrec) averaged cell by cell were as follows, from dark to 90% bleach (in ms): 1113 ± 19, 662 ± 16, 521 ± 11 and 534 ± 14. Inset: rod from best‐fitting values of r max and a in Fig. 8 A plotted as a function of the fraction bleached (F). Red curve is eqn (5) with best‐fitting value of k of 6.8. C, mean peak response amplitude (± SEM) as a function of flash intensity for the cones of Fig. 7 D–F. Colours indicate bleaching condition as follows: black, dark‐adapted (no bleaching); red, 10% bleach; green, 50% bleach; blue, 90% bleach. Data have been fitted with eqn (3) with the following values of r max (in pA) and I ½ (in P*), given in the order dark to 90% bleach: 12.4, 574; 12.4, 1000; 10.2, 1860; 6.9, 13,000. D, superimposed responses to 20 ms flashes of 5640 P* from cones of Figs 7 D–F and 8 C, normalized to same peak amplitude to illustrate acceleration of rate of response decay after bleaching. Same colour coding as in C. Best‐fitting single exponential decay time constants (τrec) averaged cell by cell were as follows, from dark to 90% bleach (in ms) 155 ± 19, 95 ± 6, 73 ± 7 and 55 ± 7. Inset: cone from best‐fitting values of r max and I ½ in Fig. 8 C plotted as a function of the fraction bleached (F). Red curve is eqn (5) with best‐fitting value of k of 4.3. [Color figure can be viewed at wileyonlinelibrary.com]
Bleaching adaptation also produced an acceleration in the rate of response decay. In Fig. 8 B and D, we show normalized responses from dark‐adapted and stably bleached rods and cones taken from the cells of Fig. 7. There was a progressive speeding of response decay much like the one we observed for photoreceptors exposed to steady background light (Figs 3 C and 5 C). Best‐fitting values of a single‐exponential time constant (τrec) to the decaying phase of the response gave a mean value for the dark‐adapted response of 1.13 s for rods and 155 ms for cones in these experiments. After bleaching 90% of the pigment, the mean value of τrec decreased to 534 ms for rods and 55 ms for cones.
For amphibian and mammalian photoreceptors, the change in sensitivity after bleaching has been shown to consist of two components. There is first a decrease in the probability of photon absorption, which is proportional to the fraction of unbleached pigment (1 – F). There is in addition a desensitization much like that in steady background light but which is produced by opsin, which can stimulate the transduction cascade with low efficiency in direct proportion to the opsin concentration (Cornwall & Fain, 1994; Cornwall et al. 1995). Bleached pigment therefore acts like a background light, with an intensity proportional to the fraction bleached.
Previous work has shown that the sum of these two effects on the sensitivity of the photoreceptor can be described by the following relation,
| (5) |
where S F is the flash sensitivity of the photoreceptor after bleaching, is the flash sensitivity in darkness before the bleach, F is the fraction bleached, and k is a constant (Jones et al. 1993, 1996; Fan et al. 2005; Nymark et al. 2012). In the insets to Fig. 8 B and D, we have plotted S F/ as a function of F fitted with eqn (5), with best‐fitting values for k of 6.8 for rods and 4.3 for cones. For comparison, similar fits for bleached photoreceptors from salamander retina have given values of k of 16.2 for rods (Jones et al. 1996) and 8.6 for cones (Jones et al. 1993).
Recovery from bleaching by regeneration of visual pigment
To show that the effects of bleaching were reversible and to demonstrate pigment regeneration in lamprey photoreceptors, we exposed bleached rods and cones to exogenous 11‐cis retinal dissolved in an ethanol/aqueous solution (see Methods). The results of these experiments are given in Fig. 9. Lamprey rods and cones showed substantial recovery of sensitivity after bleaching. Both maximum response amplitude and sensitivity reverted nearly to their values before the bleach (Fig. 9 A and C). In Fig. 9 B and D, we show normalized responses to the same flash of 117 Rh* for rods and 5640 P* for cones, before the bleach, at steady state after bleaching, and after pigment regeneration. The changes in waveform are almost completely reversed by regeneration of the bleached photopigment with 11‐cis retinal.
Discussion
Our experiments show that lamprey, whose cyclostome progenitors diverged from other vertebrates probably during the late Cambrian (Kuraku & Kuratani, 2006), have rods and cones that respond to background light and bleaches much like other vertebrates. Steady light produces an initial closing of outer‐segment channels followed by a time‐dependent reopening, and sensitivity declines according to the Weber–Fechner relation. Bright bleaching light decreases sensitivity in part by reducing the probability of photon absorption and in part by activation of the transduction cascade by bleached pigment. Both backgrounds and bleaches accelerate the rate of response decay. In the following, we show that these similarities are not superficial but detailed and pervasive. They are likely to reflect the presence early during the evolution of vertebrates of mechanisms of light adaptation much like those in existing vertebrate species.
Background adaptation
The experiments in Fig. 1 show that both rods and cones respond to light steps with two exponential time constants of decay, as also in amphibians (Fain, 1976; Matthews, 1990; Calvert et al. 2002) and mouse (Chen et al. 2010b). For rods the values of the time constants are even similar. The faster time constant is 3.3 s in Fig. 1 A, and it is 1–2 s in bullfrog (Calvert et al. 2002); in mouse it is less than half a second (Chen et al. 2010b) probably in part from the higher mammalian body temperature (Lamb, 1984; Nymark et al. 2005). The slower time constant is several tens of seconds in the rods of all of these species. In cones the time constants are much faster, in lamprey as also in other vertebrates (for example Fain et al. 1989; Matthews et al. 1990).
In darkness, both lamprey and mouse rods have responses to single photons of similar amplitude (Morshedian & Fain, 2015; Asteriti et al. 2015) and response–intensity curves of similar shape (Fig. 3 A and Woodruff et al. 2008; Chen et al. 2010a). The flash intensity required to produce a half‐maximal response is also similar. From the value of a of 0.038 for the dark‐adapted rod in Fig. 3 A, we can estimate I ½ as 18 Rh*; this is similar to reported values for mouse rods (see for example Chen et al. 2012). For dark‐adapted lamprey cones, the I ½ of 574 P* is also similar to reported values of 600–700 P* for mouse (Sakurai et al. 2011; Ingram et al. 2016). Lamprey cones are about 30 times less sensitive per photon absorbed than lamprey rods, again similar to mouse (Nikonov et al. 2006; Ingram et al. 2016). Thus the operating range of dark‐adapted lamprey rods and cones closely resembles that of other vertebrates.
Background light produces a shift in the operating range to higher intensities (Figs 3 A and 5 A), much as for photoreceptors of jawed vertebrates (see for example Kleinschmidt & Dowling, 1975; Fain, 1976; Burkhardt, 1994). The sensitivity decreases as a function of the intensity of the background according to the Weber–Fechner relation in lamprey rods and cones (Fig. 6) as in other vertebrates (Fain, 1976; Matthews et al. 1988, 1990; Mendez et al. 2001; Chen et al. 2010b; Sakurai et al. 2011). Moreover the values of the constant I 0 are similar: 67 Rh* s−1 for lamprey rods and 40–80 Rh* s−1 for mouse rods (Mendez et al. 2001; Chen et al. 2010b), 7070 P* s−1 for lamprey cones and 3000 P* s−1 for mouse (Sakurai et al. 2011). Rods in lamprey show increment saturation at an intensity of about 2000–3000 Rh* s−1, similar to the value in mouse (Chen et al. 2010b). We saw no evidence for increment saturation of lamprey cones, as has also been reported for the cones of salamander, turtle and mouse (N. T. Ingram and G. L. Fain, unpublished, and Matthews et al. 1990; Burkhardt, 1994).
Background light accelerates the decay time of lamprey rod and cone responses as in other vertebrate rods and cones (cones: Baylor & Hodgkin, 1974; Matthews et al. 1990; Tranchina et al. 1991; rods: Baylor et al. 1979; Woodruff et al. 2008). This acceleration is an essential feature of light adaptation, because it increases the sensitivity of the photoreceptors to change and motion with increasing ambient illumination. The mean time constant for lamprey rods decreased in the experiments of Figs 2 and 3 from 922 ms to 364 ms in the brightest background; the time constants for mouse rods are about three times smaller, but the extent of acceleration is also a factor of between 2 and 3 (Woodruff et al. 2008). Lamprey cone responses decay much more rapidly than lamprey rod responses, and in the brightest background the mean time constant of cone decay was only 42 ms. This rate could accommodate a flicker‐fusion frequency of 25 Hz (Dreyfert et al. 1979), which is only a factor of 2 less than our own at our considerably higher mammalian body temperature.
Bleaching adaptation
Experiments in amphibians and mammals have shown that stably bleached cells are desensitized more than would be expected from the decrease in the concentration of pigment because opsin stimulates the cascade and acts like real light to adapt the photoreceptors (Cornwall & Fain, 1994; Cornwall et al. 1995; Fan et al. 2005; Nymark et al. 2012). This is also true for lamprey: the activation of the cascade by opsin not only contributes to desensitization (Fig. 8 B and D, insets) but also to modulation of the rate of response decay (Fig. 8 B and D), which is accelerated much as in real light (Figs 3 C and 5 C). Our experiments support and extend those of Asteriti and colleagues (2015), who also observed recovery of sensitivity in bleached lamprey photoreceptors after treatment with exogenous chromophore.
By comparing desensitization produced by opsin to that produced by real light, we can estimate the efficiency of bleached pigment in stimulating the cascade. From Fig. 8 A, we calculate that a 50% bleach decreases rod sensitivity by a factor of about 0.09. After adjustment for loss of quantum catch, S F/ is equivalent to the value of S F/ produced by a real background of about 300 Rh* (Fig. 6). From the specific optical density of rod pigment of 0.005–0.01 μm−1 (Hárosi & Kleinschmidt, 1993; Morshedian et al. 2017) and the rod dimensions (Dickson & Graves, 1979; Hárosi & Kleinschmidt, 1993), there are about 2–4 × 108 pigment molecules in a lamprey rod, half as opsin at steady state after a 50% bleach. Bleached pigment is therefore about 1–3 × 10−6 times as effective at stimulating the cascade as real light. A similar calculation for lamprey cones gives a value of about 10−4. Estimates of opsin efficiency for other vertebrates are between 10−6 and 10−7 for salamander rods (Cornwall & Fain, 1994), 2 × 10−5 for salamander cones (Cornwall et al. 1995) and 2.5 × 10−5 for mouse rods (Fan et al. 2005).
Regeneration of photopigment with exogenous 11‐cis retinal produces significant recovery after bleaching (Fig. 9) as in other vertebrate rods and cones (see Fain et al. 2001). We were, however, unable to obtain complete recovery of rod sensitivity and maximum amplitude (Fig. 9 A). Although this failure could have been the result of some technical difficulty, recent experiments in mouse have shown a similar incomplete recovery of the rod response as a result of lack of dephosphorylation of bleached photopigment in isolated photoreceptors (Berry et al. 2016). It is possible that our observation in lamprey has a similar explanation.
Evolution of light adaptation
Our experiments reveal striking similarities in the behaviour of lamprey rods and cones and of the rods and cones of jawed vertebrates. The effects of background light and bleaches are nearly identical, not only in general appearance but even in quantitative detail. We were unable to identify any significant difference apart from kinetics, probably in large part attributable to differences in ambient temperature (Lamb, 1984; Nymark et al. 2005). These observations argue for the evolution of mechanisms of light adaptation at a very early stage, before the split between the cyclostomes and the rest of the vertebrates.
At present we cannot attribute these similarities to molecular pathways, in part because the molecular mechanism of vertebrate photoreceptor adaptation is controversial and still unclear (Arshavsky & Burns, 2012; Morshedian & Fain, 2014), and in part because too little is known about the biochemistry of lamprey vision. In mouse, adaptation seems to be controlled in part by regulation of cyclase via the guanylyl cyclase‐activating proteins (GCAPs) (Mendez et al. 2001), and in part by a second unidentified process which may be regulation of the phosphodiesterase (Chen et al. 2010b; Fain, 2011). Acceleration of decay rate in background light seems to be regulated by the small molecular mass Ca2+‐binding protein recoverin (Chen et al. 2012, 2015). Both GCAPs and recoverin have been tentatively identified in the lamprey genome (accession numbers S4RUK8_PETMA and S4RG87_PETMA); moreover, in preliminary experiments we have obtained evidence for expression of GCAPs and recoverin in both rods and cones with single‐cell polymerase chain reaction (PCR) using mammalian probes. It therefore seems likely that the GCAPs and recoverin are present and have a similar role in lamprey as in other vertebrates. Further exploration of the biochemistry and physiology of lamprey photoreceptors may help us understand how these proteins function and how the duplex retina evolved for visual detection with high sensitivity to contrast over a wide range of ambient illuminations.
Additional information
Competing interests
The authors declare no competing interests.
Author contributions
A.M. helped conceive and design the experiments, did the experimental work, analysed the data, and wrote a first draft of the manuscript. G.L.F. helped conceive and design the experiments, helped analyse the data, and completed the writing of the manuscript. Both authors approve of the final manuscript, are responsible for the integrity of the results, and qualify for authorship. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by National Institutes of Health Grant R01‐EY001844 and a contract from the Great Lakes Fishery Commission.
Acknowledgements
We are grateful to Michael L. Woodruff for his help in the initial stages of this research, and to Najate Aït‐Ali for assisting in preliminary experiments of rod and cone GCAP and recoverin expression.
This is an Editor's Choice article from the 15 July 2017 issue.
References
- Aguilar M & Stiles WS (1954). Saturation of the rod mechanism of the retina at high levels of stimulation. Optica Acta 1, 59–65. [Google Scholar]
- Aldridge RJ, Briggs DEG, Smith MP, Clarkson ENK & Clark NDL (1993). The anatomy of conodonts. Philos Trans R Soc Lond B Biol Sci 340, 405–421. [Google Scholar]
- Arshavsky VY & Burns ME (2012). Photoreceptor signaling: supporting vision across a wide range of light intensities. J Biol Chem 287, 1620–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asteriti S, Grillner S & Cangiano L (2015). A Cambrian origin for vertebrate rods. eLife 4, e07166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA & Hodgkin AL (1974). Changes in time scale and sensitivity in turtle photoreceptors. J Physiol 242, 729–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Lamb TD & Yau KW (1979). The membrane current of single rod outer segments. J Physiol 288, 589–611. [PMC free article] [PubMed] [Google Scholar]
- Berry J, Frederiksen R, Yao Y, Nymark S, Chen J & Cornwall C (2016). Effect of rhodopsin phosphorylation on dark adaptation in mouse rods. J Neurosci 36, 6973–6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt DA (1994). Light adaptation and photopigment bleaching in cone photoreceptors in situ in the retina of the turtle. J Neurosci 14, 1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert PD, Govardovskii VI, Arshavsky VY & Makino CL (2002). Two temporal phases of light adaptation in retinal rods. J Gen Physiol 119, 129–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter GS (1967). Structure and Habit in Vertebrate Evolution. University of Washington Press, Seattle, WA. [Google Scholar]
- Chen CK, Woodruff ML, Chen FS, Chen Y, Cilluffo MC, Tranchina D & Fain GL (2012). Modulation of mouse rod response decay by rhodopsin kinase and recoverin. J Neurosci 32, 15998–16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Woodruff ML, Chen FS, Shim H, Cilluffo MC & Fain G (2010a). Replacing the rod with the cone transducin alpha subunit decreases sensitivity and accelerates response decay. J Physiol 588, 3231–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Woodruff ML & Fain GL (2015). Rhodopsin kinase and recoverin modulate phosphodiesterase during mouse photoreceptor light adaptation. J Gen Physiol 145, 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Woodruff ML, Wang T, Concepcion F, Tranchina D & Fain GL (2010b). Channel modulation and the mechanism of light adaptation in mouse rods. J Neurosci 30, 16232–16240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall MC & Fain GL (1994). Bleached pigment activates transduction in isolated rods of the salamander retina. J Physiol 480, 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall MC, Matthews HR, Crouch RK & Fain GL (1995). Bleached pigment activates transduction in salamander cones. J Gen Physiol 106, 543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DH & Graves DA (1979). Fine structure of the lamprey photoreceptors and retinal pigment epithelium (Petromyzon marinus L.). Exp Eye Res 29, 45–60. [DOI] [PubMed] [Google Scholar]
- Dreyfert T, Holmberg K & Struwe G (1979). Critical flicker fusion frequency of the river lamprey (Lampetra fluviatilis). Vision Res 19, 551–553. [DOI] [PubMed] [Google Scholar]
- Fain GL (1976). Sensitivity of toad rods: Dependence on wave‐length and background illumination. J Physiol 261, 71–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL (2003). Sensory Transduction. Sinauer, Inc, Sunderland, MA. [Google Scholar]
- Fain GL (2011). Adaptation of mammalian photoreceptors to background light: putative role for direct modulation of phosphodiesterase. Mol Neurobiol 44, 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Lamb TD, Matthews HR & Murphy RL (1989). Cytoplasmic calcium as the messenger for light adaptation in salamander rods. J Physiol 416, 215–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Matthews HR, Cornwall MC & Koutalos Y (2001). Adaptation in vertebrate photoreceptors. Physiol Rev 81, 117–151. [DOI] [PubMed] [Google Scholar]
- Fan J, Woodruff ML, Cilluffo MC, Crouch RK & Fain GL (2005). Opsin activation of transduction in the rods of dark‐reared Rpe65 knockout mice. J Physiol 568, 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen R, Boyer NP, Nickle B, Chakrabarti KS, Koutalos Y, Crouch RK, Oprian D & Cornwall MC (2012). Low aqueous solubility of 11‐cis‐retinal limits the rate of pigment formation and dark adaptation in salamander rods. J Gen Physiol 139, 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govardovskii VI & Lychakov DV (1984). Visual cells and visual pigments of the lamprey, Lampetra fluviatilis . J Comp Physiology A 154, 279–286. [Google Scholar]
- Hárosi FI & Kleinschmidt J (1993). Visual pigments in the sea lamprey, Petromyzon marinus . Vis Neurosci 10, 711–715. [DOI] [PubMed] [Google Scholar]
- Ingram NT, Sampath AP & Fain GL (2016). Why are rods more sensitive than cones? J Physiol 594, 5415–5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GJ, Cornwall MC & Fain GL (1996). Equivalence of background and bleaching desensitization in isolated rod photoreceptors of the larval tiger salamander. J Gen Physiol 108, 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GJ, Fein A, MacNichol EF Jr & Cornwall MC (1993). Visual pigment bleaching in isolated salamander retinal cones. Microspectrophotometry and light adaptation. J Gen Physiol 102, 483–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J & Dowling JE (1975). Intracellular recordings from gecko photoreceptors during light and dark adaptation. J Gen Physiol 66, 617–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraku S & Kuratani S (2006). Time scale for cyclostome evolution inferred with a phylogenetic diagnosis of hagfish and lamprey cDNA sequences. Zoolog Sci 23, 1053–1064. [DOI] [PubMed] [Google Scholar]
- Lamb TD (1984). Effects of temperature changes on toad rod photocurrents. J Physiol 346, 557–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, McNaughton PA & Yau KW (1981). Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol 319, 463–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, Patel H, Chuah A, Natoli RC, Davies WI, Hart NS, Collin SP & Hunt DM (2016). Evolution of vertebrate phototransduction: cascade activation. Mol Biol Evol 33, 2064–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HR ( 1990). Messengers of transduction and adaptation in vertebrate photoreceptors In Light and Life in the Sea, ed. Herring PJ, Campbell M, Whitfield M. & Maddock L, pp. 185–198. Cambridge University Press, Cambridge. [Google Scholar]
- Matthews HR, Fain GL, Murphy RL & Lamb TD (1990). Light adaptation in cone photoreceptors of the salamander: a role for cytoplasmic calcium. J Physiol 420, 447–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HR, Murphy RL, Fain GL & Lamb TD (1988). Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature 334, 67–69. [DOI] [PubMed] [Google Scholar]
- Mendez A, Burns ME, Sokal I, Dizhoor AM, Baehr W, Palczewski K, Baylor DA & Chen J (2001). Role of guanylate cyclase‐activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc Natl Acad Sci USA 98, 9948–9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedian A & Fain GL (2014). Molecular mechanism of adaptation in vertebrate rods In Vertebrate Photoreceptors: Functional Molecular Bases, ed. Furukawa T, Hurley JB. & Kawamura S, pp. 73–90. Springer, Berlin. [Google Scholar]
- Morshedian A & Fain GL (2015). Single‐photon sensitivity of lamprey rods with cone‐like outer segments. Curr Biol 25, 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedian A, Toomey MB, Pollack GE, Frederiksen R, Enright JM, McCormick SD, Cornwall MC, Fain GL & Corbo JC (2017). Cambrian origin of the CYP27C1‐mediated vitamin A1‐to‐vitamin A2 switch, a key mechanism of vertebrate sensory plasticity. Royal Society Open Science, in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muradov H, Boyd KK, Kerov V & Artemyev NO (2007). PDE6 in lamprey Petromyzon marinus: implications for the evolution of the visual effector in vertebrates. Biochemistry 46, 9992–10000. [DOI] [PubMed] [Google Scholar]
- Muradov H, Kerov V, Boyd KK & Artemyev NO (2008). Unique transducins expressed in long and short photoreceptors of lamprey Petromyzon marinus . Vision Res 48, 2302–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov SS, Kholodenko R, Lem J & Pugh EN Jr (2006). Physiological features of the S‐ and M‐cone photoreceptors of wild‐type mice from single‐cell recordings. J Gen Physiol 127, 359–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nymark S, Frederiksen R, Woodruff ML, Cornwall MC & Fain GL (2012). Bleaching of mouse rods: microspectrophotometry and suction‐electrode recording. J Physiol 590, 2353–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nymark S, Heikkinen H, Haldin C, Donner K & Koskelainen A (2005). Light responses and light adaptation in rat retinal rods at different temperatures. J Physiol 567, 923–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani D, Mohun SM, Harris SR, McInerney JO & Wilkinson M (2006). Molecular evidence for dim‐light vision in the last common ancestor of the vertebrates. Curr Biol 16, R318–R319; author reply R320. [DOI] [PubMed] [Google Scholar]
- Poliakov E, Gubin AN, Stearn O, Li Y, Campos MM, Gentleman S, Rogozin IB & Redmond TM (2012). Origin and evolution of retinoid isomerization machinery in vertebrate visual cycle: hint from jawless vertebrates. PloS One 7, e49975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K, Chen J & Kefalov VJ (2011). Role of guanylyl cyclase modulation in mouse cone phototransduction. J Neurosci 31, 7991–8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K, Chen J, Khani SC & Kefalov VJ (2015). Regulation of mammalian cone phototransduction by recoverin and rhodopsin kinase. J Biol Chem 290, 9239–9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom IJ, Smith MM & Smith MP (2001). The Ordovician radiation of vertebrates In Major Events in Early Vertebrate Evolution, ed. Ahlberg PE, pp. 156–171. Taylor and Francis, London. [Google Scholar]
- Smith MP, Sansom IJ & Cochrane KD (2001). The Cambrian origin of vertebrates In Major Events in Early Vertebrate Evolution, ed. Ahlberg PE, pp. 67–84. Taylor and Francis, London. [Google Scholar]
- Tranchina D, Sneyd J & Cadenas ID (1991). Light adaptation in turtle cones. Testing and analysis of a model for phototransduction. Biophys J 60, 217–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff ML, Janisch KM, Peshenko IV, Dizhoor AM, Tsang SH & Fain GL (2008). Modulation of phosphodiesterase6 turnoff during background illumination in mouse rod photoreceptors. J Neurosci 28, 2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff ML, Lem J & Fain GL (2004). Early receptor current of wild‐type and transducin knockout mice: photosensitivity and light‐induced Ca2+ release. J Physiol 557, 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H & Yokoyama S (1997). Molecular evolution of the rhodopsin gene of marine lamprey, Petromyzon marinus . Gene 191, 1–6. [DOI] [PubMed] [Google Scholar]


