Abstract
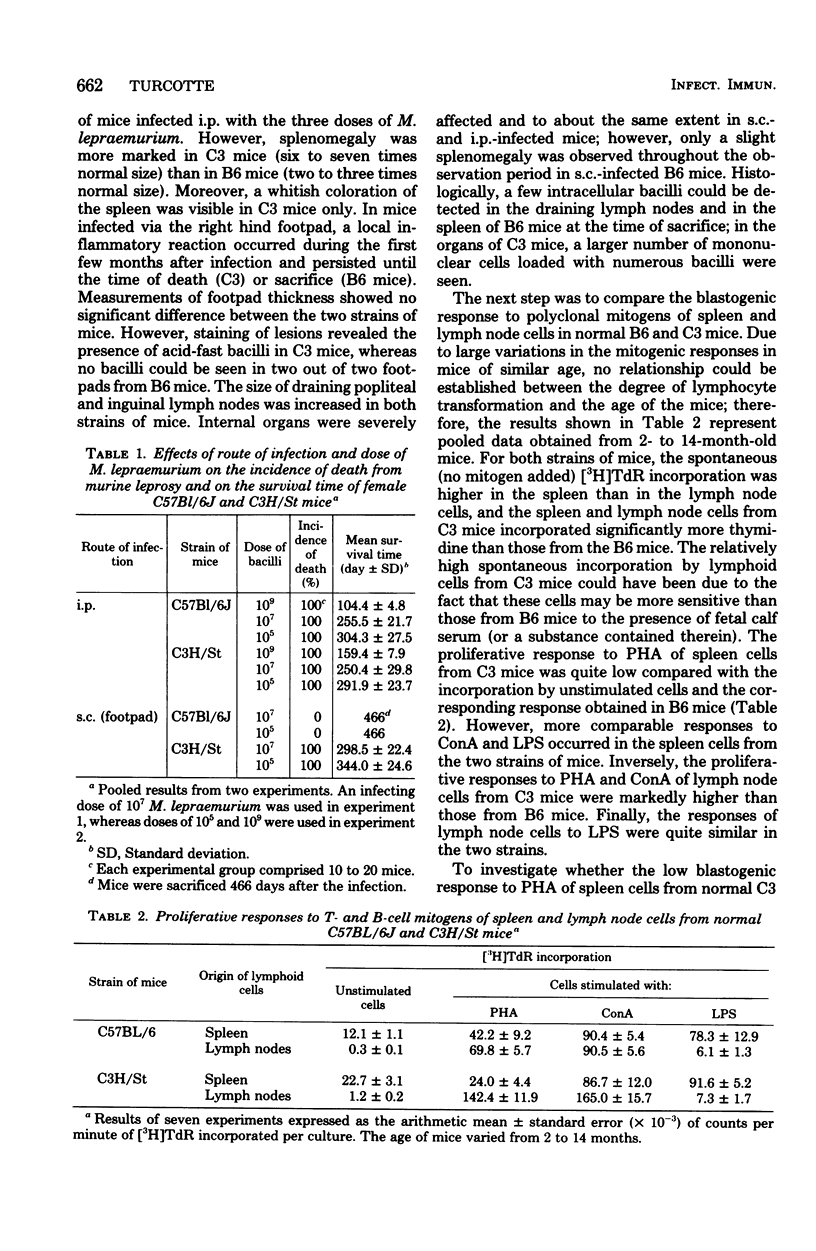
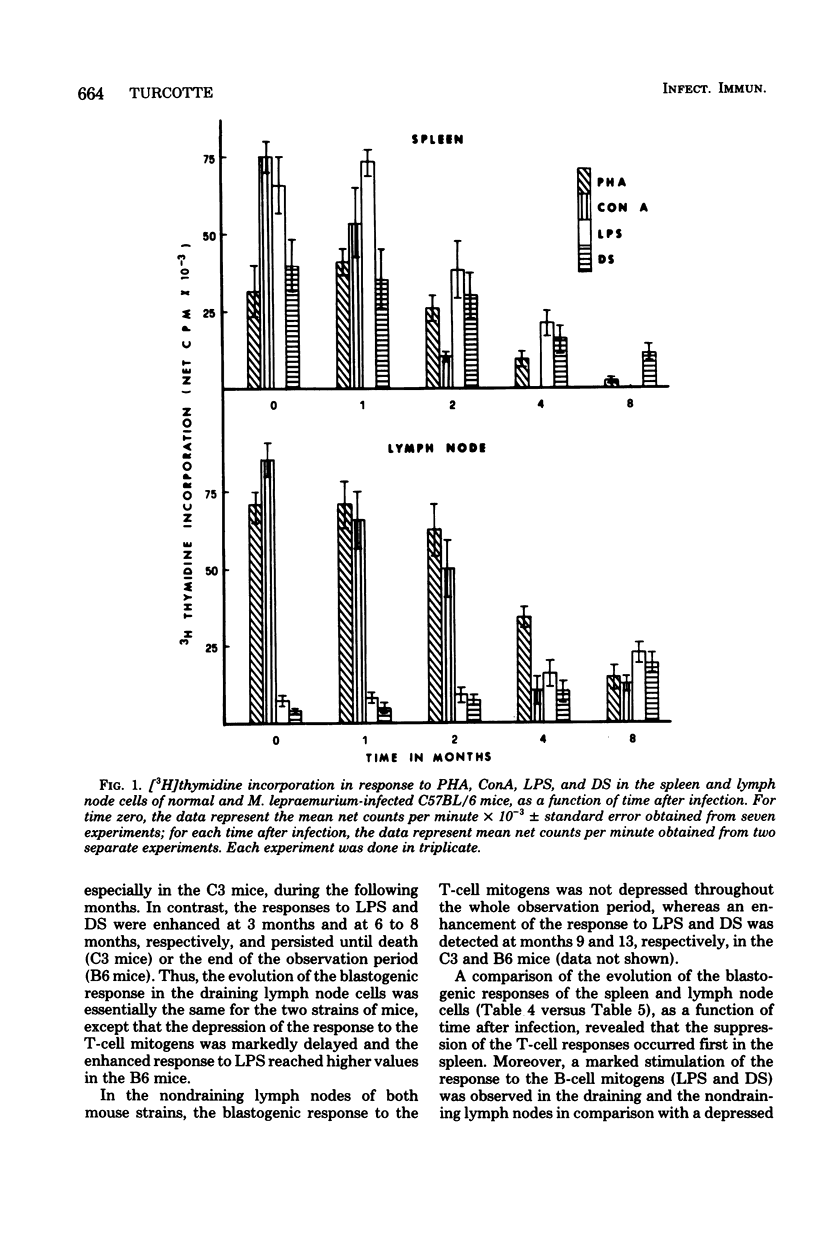
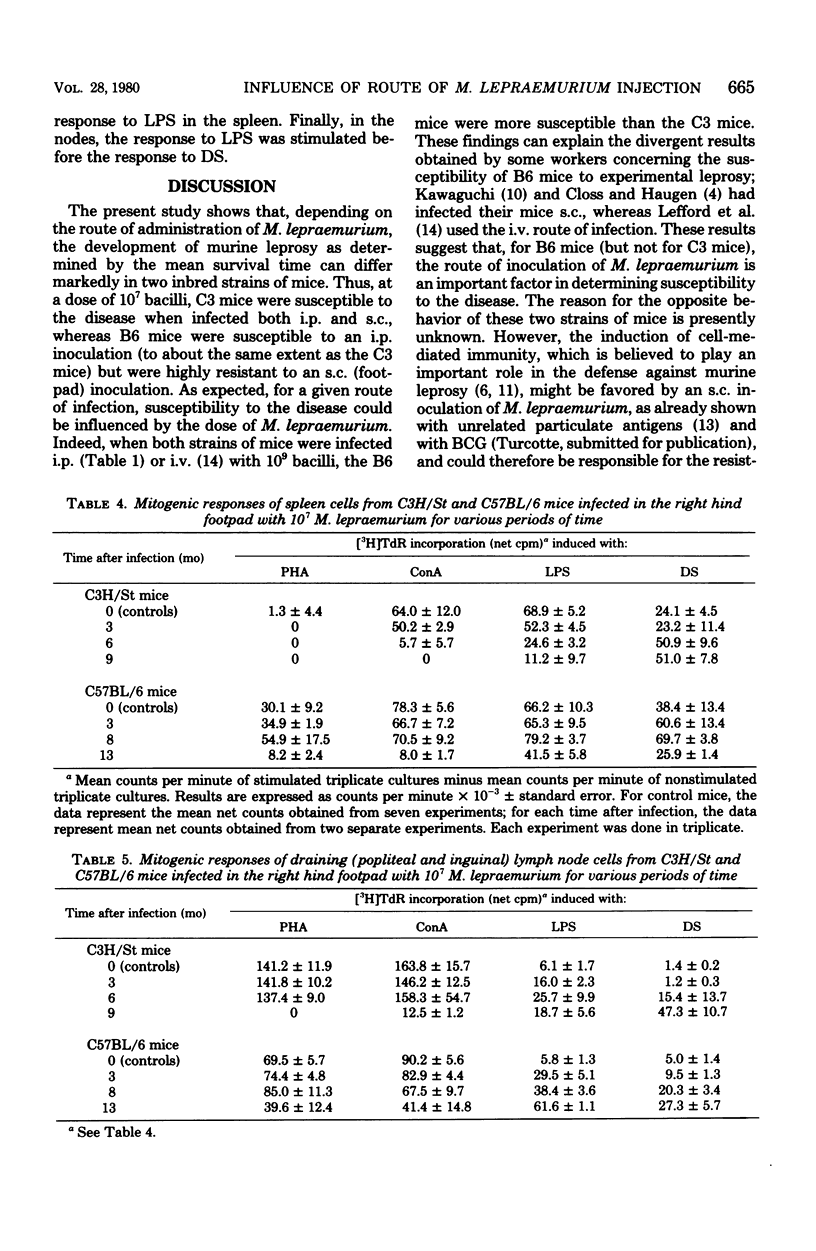
Groups of female C57BL/6 and C3H/St mice were inoculated intraperitoneally (i.p.) with 109, 107, and 105 bacilli and into the right hind footpad with 107 and 105 bacilli of Mycobacterium lepraemurium. The incidence of death from leprosy and the mean survival time of leprous mice were recorded. In addition, the blastogenic responses to the T-cell mitogens phytohemagglutinin and concanavalin A and to the B-cell mitogens lipopolysaccharide and dextran sulfate were evaluated at various times during the course of infection in the spleen and peripheral lymph nodes of mice infected with 107 bacilli. When M. lepraemurium was administered i.p., the two strains of mice succumbed to the disease at about the same time, except for the C57BL/6 mice infected with 109 bacilli, which died earlier than the C3H/St mice. Moreover, in both strains of mice, a progressive depression of blastogenesis, first to the T-cell mitogens and then to the B-cell mitogens in the spleen, and to the T-cell mitogens in the peripheral lymph nodes, occurred during the course of the infection, whereas the response to the B-cell mitogens in the nodes increased slowly during the advanced stage of the disease. When 107 and 105 bacilli were injected into the footpad, the C3H/St mice succumbed to the disease at 298 and 344 days, respectively, and the modifications of blastogenesis were similar to those observed in i.p.-infected C3H/St mice. In contrast, the C57BL/6 mice appeared resistant to footpad inoculation of M. lepraemurium, since they lived until the end of the observation period (466 days postinfection) and the depression of blastogenesis was not detectable until 1 year after the infection. Thus, it is concluded that for the C57BL/6 mice (but not for the C3H/St mice), the route of administration of M. lepraemurium can markedly influence the susceptibility or resistance to leprosy.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M. J., Epstein R., Cohn M. The effect of 2-mercaptoethanol on murine mixed lymphocyte cultures. J Exp Med. 1974 Apr 1;139(4):1025–1030. doi: 10.1084/jem.139.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Carlson E. M., Gershon R. K. The evolution of immunosuppressive cell populations in experimental mycobacterial infection. J Immunol. 1978 May;120(5):1709–1716. [PubMed] [Google Scholar]
- Bullock W. E., Jr Perturbation of lymphocyte circulation in experimental murine leprosy. I. Description of the defect. J Immunol. 1976 Oct;117(4):1164–1170. [PubMed] [Google Scholar]
- Closs O. Experimental murine leprosy: growth of Mycobacterium lepraemurium in C3H and C57/BL mice after footpad inoculation. Infect Immun. 1975 Sep;12(3):480–489. doi: 10.1128/iai.12.3.480-489.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O., Haugen O. A. Experimental murine leprosy. 3. Early local reaction to mycobacterium lepraemurium in C3H and C57/BL mice. Acta Pathol Microbiol Scand A. 1975 Jan;83(1):51–58. [PubMed] [Google Scholar]
- Closs O., Haugen O. A. Experimental murine leprosy. 4. The gross appearance and microscopic features of the local infiltrate after subcutaneous inoculation of C3H and C57/BL mice with mycobacterium lepraemurium. Acta Pathol Microbiol Scand A. 1975 Jan;83(1):59–68. [PubMed] [Google Scholar]
- Folch H., Waksman B. H. Regulation of lymphocyte responses in vitro. V. Suppressor activity of adherent and nonadherent rat lymphoid cells. Cell Immunol. 1973 Oct;9(1):12–24. doi: 10.1016/0008-8749(73)90163-9. [DOI] [PubMed] [Google Scholar]
- Gery I., Navok T., Stupp Y. Selective accumulation of cells with 'B' properties in stimulated lymph nodes. Immunology. 1977 Nov;33(5):727–731. [PMC free article] [PubMed] [Google Scholar]
- KAWAGUCHI Y. Classification of mouse leprosy. Jpn J Exp Med. 1959 Dec;29:651–663. [PubMed] [Google Scholar]
- Kawaguchi Y., Matsuoka M., Kawatsu K., Homma J. Y., Abe C. Susceptibility to murine leprosy bacilli of nude mice. Jpn J Exp Med. 1976 Jun;46(3):167–180. [PubMed] [Google Scholar]
- Keller R. Major changes in lymphocyte proliferation evoked by activated macrophages. Cell Immunol. 1975 Jun;17(2):542–551. doi: 10.1016/s0008-8749(75)80058-x. [DOI] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Influence of dose and route of antigen injection on the immunological induction of T cells. J Exp Med. 1974 Mar 1;139(3):528–542. doi: 10.1084/jem.139.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J., Patel P. J., Poulter L. W., Mackaness G. B. Induction of cell-mediated immunity to Mycobacterium lepraemurium in susceptible mice. Infect Immun. 1977 Dec;18(3):654–659. doi: 10.1128/iai.18.3.654-659.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R. M., Tokunaga T. Strain difference of delayed-type hypersensitivity to BCG and its genetic control in mice. Infect Immun. 1978 Dec;22(3):657–664. doi: 10.1128/iai.22.3.657-664.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novogrodsky A., Stenzel K. H., Rubin A. L. Stimulation of human peripheral blood lymphocytes by periodate, galactose oxidase, soybean agglutinin, and peanut agglutinin: differential effects of adherent cells. J Immunol. 1977 Mar;118(3):852–857. [PubMed] [Google Scholar]
- Press O. W., Rosse C., Clagett J. The distribution of rapidly and slowly renewed T, B, and "null" lymphocytes in mouse bone marrow, thymus, lymph nodes, and spleen. Cell Immunol. 1977 Sep;33(1):114–124. doi: 10.1016/0008-8749(77)90139-3. [DOI] [PubMed] [Google Scholar]
- Rojas-Espinosa O., Casoluengo-Méndez M., Díaz G. V. Antibody-mediated immunity in CFW mice infected with Mycobacterium lepraemurium. Humoral immune response in murine leprosy. Clin Exp Immunol. 1976 Dec;26(3):381–387. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., King J. Is the adherent, non-specific 'suppressor' cell from rodent spleens an in vitro artefact? Clin Exp Immunol. 1978 Jun;32(3):466–470. [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., Farrar J. J., Dougherty S. Absolute macrophage dependency of T lymphocyte activation by mitogens. J Immunol. 1976 Jan;116(1):131–139. [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968 Jan-Mar;36(1):78–82. [PubMed] [Google Scholar]
- Stobo J. D., Paul W. E. Functional heterogeneity of murine lymphoid cells. 3. Differential responsiveness of T cells to phytohemagglutinin and concanavalin A as a probe for T cell subsets. J Immunol. 1973 Feb;110(2):362–375. [PubMed] [Google Scholar]
- Turcotte R., Lafleur L., Labrèche M. Opposite effects of BCG on spleen and lymph node cells: lymphocyte proliferation and immunoglobulin synthesis. Infect Immun. 1978 Sep;21(3):696–704. doi: 10.1128/iai.21.3.696-704.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R. Suppressor cells in experimental murine leprosy. Int J Lepr Other Mycobact Dis. 1978 Jul-Dec;46(3-4):358–363. [PubMed] [Google Scholar]
- Turk J. L., Bryceson A. D. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]
- Watson S. R., Sljivić V. S., Brown I. N. Defect of macrophage function in the antibody response to sheep erythrocytes in systemic Mycobacterium lepraemurium infection. Nature. 1975 Jul 17;256(5514):206–208. doi: 10.1038/256206b0. [DOI] [PubMed] [Google Scholar]


