Abstract
Aims
In vitro studies have demonstrated that formation of reactive oxygen species (ROS) contributes to the effect of bactericidal antibiotics. The formation of ROS is not restricted to bacteria, but also occurs in mammalian cells. Oxidative stress is linked to several diseases. This study investigates whether antibiotic drugs induce oxidative stress in healthy humans as a possible mechanism for adverse reactions to the antibiotic drugs.
Methods
This study contains information from two randomised, controlled trials. Participants underwent 1 week treatment with clarithromycin, trimethoprim, phenoxymethylpenicillin (penicillin V), or placebo. Oxidative modifications were measured as 24‐h urinary excretion of 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine (8‐oxodG) and 8‐oxo‐7,8‐dihydroguanosine (8‐oxoGuo), and plasma levels of malondialdehyde before and after treatment as a measurement of DNA oxidation, RNA oxidation, and lipid peroxidation, respectively.
Results
Clarithromycin significantly increased urinary excretion of 8‐oxodG by 22.0% (95% confidence interval (CI): 3.6–40.4%) and 8‐oxoGuo by 14.9% (95% CI: 3.7–26.1%). Further, we demonstrated that trimethoprim significantly lowered urinary excretion of 8‐oxodG by 21.7% (95% CI: 5.8–37.6%), but did not influence urinary excretion of 8‐oxoGuo. Penicillin V did not influence urinary excretion of 8‐oxodG or 8‐oxoGuo. None of the antibiotic drugs influenced plasma levels of malondialdehyde.
Conclusion
Clarithromycin significantly increases oxidative nucleic acid modifications. Increased oxidative modifications might explain some of clarithromycin's known adverse reactions. Trimethoprim significantly lowers DNA oxidation but not RNA oxidation. Penicillin V had no effect on oxidative nucleic acid modifications.
Keywords: antibiotics; oxidative stress; 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine; 8‐oxo‐7,8‐dihydroguanosine; DNA oxidation; RNA oxidation
What is Already Known about this Subject
Formation of reactive oxygen species contributes to the effect of bactericidal antibiotics. The formation of reactive oxygen species is not restricted to bacteria, but also occurs in mammalian cells.
Oxidative stress is associated with several diseases e.g. type 2 diabetes, neurodegenerative‐, and cardiovascular diseases.
What this Study Adds
Clarithromycin significantly increases oxidative nucleic acid modifications in humans. Clinical studies are needed to establish whether clarithromycin has safety issues in specific patient populations.
Trimethoprim significantly lowers DNA oxidation but not RNA oxidation.
Penicillin V has no effect on oxidative nucleic acid modifications.
Introduction
Antibiotic drugs are widely used against various infections, are classified according to their accepted mechanism of action, and are traditionally grouped in bacteriostatic or bactericidal antibiotics. Bacteriostatic antibiotics prevent growth of bacteria while bactericidal antibiotics kill bacteria 1.
In addition to the accepted mechanisms of bactericidal antibiotic drugs, it has been demonstrated that formation of reactive oxygen species (ROS) contributes to the bactericidal effect, irrespective of their targets 2, 3, 4, 5, 6, 7, 8, 9. The production of ROS is thought to be from activation of the tricarboxylic acid cycle and thereby stimulation of the electron chain 2. However, there is still no agreement on the clinical relevance of the ROS production 10, 11. The formation of ROS implies a possible target effect of antibiotic drugs, and furthermore raises the question of a possible mechanism for adverse reactions (AR). It has been demonstrated that bactericidal antibiotics induce ROS formation not only in bacteria but also in mammalian cells 12, 13. Oxidative modifications are linked to ageing 14 and several diseases e.g. type 2 diabetes (T2D) 15, neurodegenerative diseases 16, 17, 18, and cardiovascular diseases 19. The elevated levels of oxidative modifications are considered prognostic for mortality in patients with T2D 20, 21 and also associated with development of lung cancer in non‐smokers 22 and oestrogen receptor positive breast cancer 23. However, it remains a subject of great interest whether the elevated levels of oxidative modifications are prognostic in neurodegenerative‐ and cardiovascular diseases 24. It is possible that elevated levels of oxidative modifications may contribute to drug AR; for example, the finding that 2 weeks’ treatment with clarithromycin significantly increases cardiovascular disease three years after treatment in patients with stable coronary heart diseases 25.
Several studies have found an association between bactericidal antibiotic drugs and the production of ROS 2, 3, 4, 5, 6, 7, 8, 9, 12, 13, but this has not been found for bacteriostatic antibiotic drugs 2, 3, 12, 13.
The aim of this study is to evaluate the effect of clarithromycin, trimethoprim, and phenoxymethylpenicillin (penicillin V) on oxidative modifications in healthy humans. Clarithromycin and trimethoprim are both classified as bacteriostatic antibiotic drugs, whereas penicillin V is a bactericidal antibiotic drug 1.
We measured three markers of oxidative modifications in order to differentiate the effects in various cellular compartments. We used 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine (8‐oxodG), 8‐oxo‐7,8‐dihydroguanosine (8‐oxoGuo), and malondialdehyde (MDA) as measurement of intranuclear‐ (DNA oxidation), cytosolic‐ (RNA oxidation), and plasma oxidative modifications (lipid peroxidation), respectively 26, 27. The measurements were conducted before and after exposure to the three antibiotics and placebo.
Methods
The present study provides information from two trials; CLAROX (EudraCT: 2008‐001299‐61; ClinicalTrials.gov: NCT00707330) and PENTRIOX (EudraCT: 2010‐022762‐27; ClinicalTrials.gov: NCT02188472). Both trials have the same inclusion criteria, study outcome, and sample size. The trials are designed as randomised, controlled with cross‐over or parallel groups, respectively.
Trial design
CLAROX
CLAROX is a randomised, controlled, cross‐over study, where participants in random order received no treatment or active treatment for 1 week separated by a 2‐week washout period.
The study has an open‐label design to diminish logistic and cost. The laboratory analyses were performed blinded to treatment information.
Trial medicine was obtained from the Pharmacy of the Capital Region of Denmark.
The trial design is illustrated in supplementary information. Fasting participants underwent five visits at the trial site after informed consent. (i) A screening visit to ensure the participants eligibility to the trial. A blood sample and basic clinical tests including measurement of blood pressure, pulse, height, weight and an electrocardiogram (ECG) were recorded. (ii) At the second visit, trial medicine was dispensed to the participants. A 24‐h urine sample and a blood sample were collected. (iii) After the first treatment schedule, the third visit took place. Participants delivered a 24‐h urine sample, and a blood sample was collected. (iv) Before the second treatment schedule, participants underwent a fourth visit. Blood pressure and an ECG were recorded, and a blood sample was collected. (v) At the fifth and final visit, participants delivered a 24‐h urine sample, excess trial medicine, and a blood sample was collected.
The trial was approved by the Regional Committee on Biomedical Research Ethics (H‐D‐2008‐026) and the Danish Health and Medicines Authority. The trial was conducted in accordance with the Declaration of Helsinki.
PENTRIOX
PENTRIOX is a double‐blinded, randomised, placebo‐controlled study with three parallel groups. The treatment arms consisted of treatment with trimethoprim, penicillin V, and placebo, each for 1 week.
Trial medicine was randomised and labelled by the Pharmacy of the Capital Region of Denmark.
The trial design is illustrated in supplementary information. Non‐fasting participants underwent three visits at the trial site after informed consent. (i) A screening visit to ensure the participants eligibility to the trial. A blood sample and basic clinical tests including measurements of blood pressure, pulse, height, weight and an ECG were recorded. (ii) At the start visit, trial medicine was dispensed to participants. A 24‐h urine sample and a blood sample were collected. (iii) At the third and final visit, participants delivered a 24‐h urine sample, excess trial medicine and a blood sample was collected.
The trial was approved by the Regional Committee on Biomedical Research Ethics (H‐1‐2010‐099) and the Danish Health and Medicines Authority. The trial was conducted in accordance with the Declaration of Helsinki.
Participants
In both CLAROX and PENTRIOX, the trial population consisted of healthy, Caucasian males who were non‐smokers with a body mass index >18 kg m2 and <30 kg m2. The exclusion criteria are available in supplementary information, and were in general selected to ensure the safety of participants as well as avoid possible influence on the primary outcome, oxidative nucleic acid modifications.
Participants were recruited through approved advertisement in local media, online, and in public places. Potential participants who met the inclusion criteria received written information in the form of the approved informed consent.
To ensure adherence, participants received a text message as a reminder of the medicine intake. The participants answered the text message after medicine intake, hereby confirming adherence. Satisfactory adherence level of 65% was predefined in the protocol. Returned medication was destroyed at the trial site.
Participants were informed prior to the trial about possible side effects. They were told to contact an investigator if they experienced any side effect. Adverse events were registered after each treatment schedule. All adverse events were reported at the end of the trials to the Danish Health and Medicines Authority and the Danish National Committee on Biomedical Research Ethics.
Intervention
CLAROX participants received 1 tablet clarithromycin (Klacid Uno; BGP Products, Maidenhead, UK) 500 mg once daily for 1 week. The trial medicine was administrated with a meal in the morning. Participants in the control schedule received no treatment.
PENTRIOX participants received two tablets: 330 mg penicillin V (Pancillin; Sandoz, Holzkirchen, Germany), 100 mg trimethoprim (Trimopan; Orion Pharma, Espoo, Finland), or placebo twice daily for 1 week. The trial medicine was administrated with a meal in the morning and in the evening.
All trial medicine was administered in clinically relevant dosages 28, 29, 30, and complied with GMP 31 and GCP regulations 32.
Study outcomes
Primary outcomes
The primary outcomes were urinary excretion of 8‐oxodG and 8‐oxoGuo as a measurement of whole body DNA‐ and RNA oxidation, respectively 26.
The 24‐h urine collection was sampled from 24 h prior to the second, third and fifth visit in CLAROX and prior to the second and third visit in PENTRIOX. The volume urine collected in each container was recorded, well mixed and an aliquot was stored at –20°C until analysis.
If participants recorded loss of a urine void, an estimated value was added to the recorded diuresis. Satisfactory adherence level of 75% was predefined in the protocol.
The analysis was done simultaneous by tandem mass spectrometry with isotope dilution, details and quality control are reported elsewhere 33. As mentioned by Henriksen et al. 33, the lower limit of quantification for both 8‐oxodG and 8‐oxoGuo was 1.0 nmol l–1. The average within‐day precision (percentage of relative standard deviation) was 3.7 and 4.4%, and the average between‐day precision was 2.3 and 4.0% for 8‐oxodG and 8‐oxoGuo, respectively. The accuracy (percentage recovery) was 106.9 and 106.2% for 8‐oxodG and 8‐oxoGuo, respectively 33.
Secondary outcome
The secondary outcome was plasma levels of MDA as a measurement of lipid peroxidation 27.
Blood samples were collected in 10‐mL heparin prepared utensils. The samples were centrifuged for 10 min and plasma was transferred to three sample vials and stored at –80°C until analysis. Details of sample preparation, analysis and quality control are reported elsewhere 34. In brief, plasma samples were treated with phosphotungstic acid to remove other compounds that may interfere with the assay. Subsequently, butylated hydroxytoluene, a chain‐breaking antioxidant that prevent peroxidation during the assay itself, was added, and MDA reacted with thiobarbituric acid (TBA 2.3 μmol l–1) in the presence of acetic acid (1.75 mol l–1) for 1 h at 95°C to form the MDA(TBA)2 fluorescent adduct. Following extraction with butanol, the genuine MDA(TBA)2 adduct was selectively quantified by high‐performance liquid chromatography (HPLC) with fluorescence detection (excitation, 515 nm; emission, 553 nm). The limit of quantification was 0.1 μmol l–1 MDA and the within‐ and between‐day coefficients of variation for the entire assay were 8.2% and 14.1%, respectively 34. The analyses were performed at Department of Veterinary Pathobiology, University of Copenhagen.
Pretrial power calculation
With 10% coefficient of variation a sample size of 25 participants was estimated to provide 80% power, and to detect 8% increase in oxidative nucleic acid modifications at 5% significance.
Due to possible dropout, it was decided to include 30 participants in each trial.
Statistics
Data management and analysis were performed using R version 3.2.2 35.
Data was analysed per‐protocol. Clinical and demographic characteristics were analysed using a Student t test in CLAROX and one‐way ANOVA test in PENTRIOX.
Primary and secondary outcome were analysed using a paired t test in CLAROX and Student t test in PENTRIOX. Data were investigated for normal distribution and verified using a non‐parametric test.
A two‐sided P‐value < 0.05 was considered statistically significant.
Results
Study population and flow
CLAROX
Participant flow is illustrated in Figure 1. Twenty‐eight potential participants were assessed for eligibility. Two potential participants were excluded by investigators due to health issue: chronic inflammatory disease (one participant) and cardiac disease (one participant). Twenty‐six participants were randomised. One participant was withdrawn from treatment due to penicillin treatment of a skin infection. Twenty‐five participants successfully completed the trial and their urine‐ and blood samples were analysed.
Figure 1.
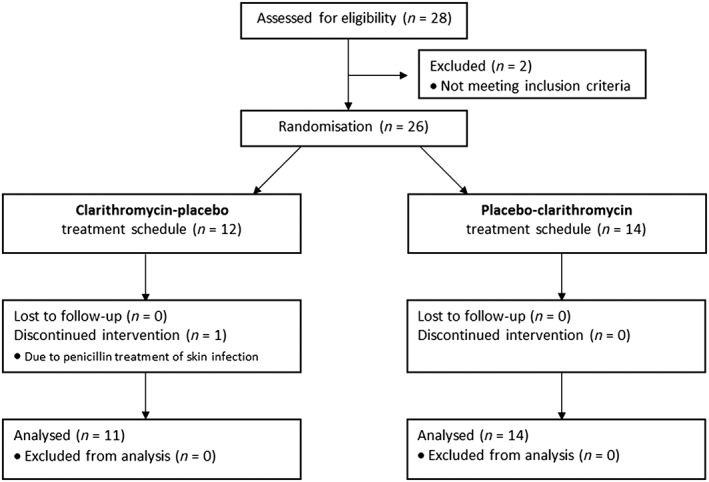
Participants flow for the CLAROX trial
Baseline clinical and biochemistry characteristics of the participants are presented in Table 1. The characteristics were within normal range and did not differ between groups, except diastolic blood pressure which was significantly lower in the group that received clarithromycin treatment followed by control treatment (P = 0.02).
Table 1.
Baseline clinical and biochemistry characteristics (CLAROX)
| Clarithromycin–control | Control–clarithromycin | P a | |
|---|---|---|---|
| n | 11 | 14 | |
| Age (years) | 24.2 (3.0) | 24.3 (2.0) | NS |
| Weight (kg) | 78.8 (5.4) | 77.6 (6.2) | NS |
| Height (m) | 1.84 (0.05) | 1.84 (0.05) | NS |
| Body mass index (kg m –2 ) | 23.4 (1.7) | 22.9 (1.6) | NS |
| Systolic blood pressure (mmHg) | 127.2 (9.2) | 126.7 (11.9) | NS |
| Diastolic blood pressure (mmHg) | 73.8 (4.8) | 79.3 (5.6) | 0.02 |
| Pulse (beats min –1 ) | 61.7 (8.8) | 62.0 (11.3) | NS |
| QTc interval (ms) | 384.7 (32.3) | 393.0 (31.1) | NS |
| Total cholesterol (mmol l –1 ) | 3.8 (0.6) | 4.1 (0.7) | NS |
| HDL cholesterol (mmol l –1 ) | 1.5 (0.3) | 1.5 (0.3) | NS |
| LDL cholesterol (mmol l –1 ) | 2.2 (0.6) | 2.4 (0.5) | NS |
| Triglycerides (mmol l –1 ) | 0.8 (0.4) | 0.8 (0.3) | NS |
| Serum creatinine (mmol l –1 ) | 78.8 (8.0) | 79.3 (8.4) | NS |
| Ferritin (μmol l –1 ) | 131.5 (53.2) | 110.3 (61.6) | NS |
| Iron (μmol l –1 ) | 19.4 (7.6) | 22.0 (11.8) | NS |
| Transferrin saturation | 0.29 (0.11) | 0.31 (0.17) | NS |
| Potassium (mmol l –1 ) | 4.0 (0.2) | 4.0 (0.2) | NS |
| Magnesium (mmol l –1 ) | 0.9 (0.1) | 0.9 (0.1) | NS |
Values are presented as mean (standard deviation)
Student t test; NS = not statistically significant.
There was no significant difference in baseline urinary excretion of 8‐oxodG (P = 0.10) or 8‐oxoGuo (P = 0.44) between the groups.
According to registration by trial participants, the overall adherence of urine samples was 93%. According to a control system based on phone text messages from participants, adherence of medicine intake was 99%.
Twelve participants reported a total of 13 adverse events (AE). Nine were evaluated to be related to the medicine (AR), none were serious AE. Gastrointestinal reactions and nausea were the most frequently observed events.
PENTRIOX
Participant flow is illustrated in Figure 2. One hundred and twelve potential participants were assessed for eligibility. Twenty‐two of the potential participants were excluded at screening visit due to: hypercholesterolaemia (11 participants); elevated blood pressure (two participants); elevated ALAT (two participants); isolated hypertriglyceridaemia (one participant); hypopotassaemia (one participant); previous heart arrhythmia (one participant); murmur of the heart (one participant); use of illegal drugs (one participant); and withdrawal for no given reason (two participants).
Figure 2.
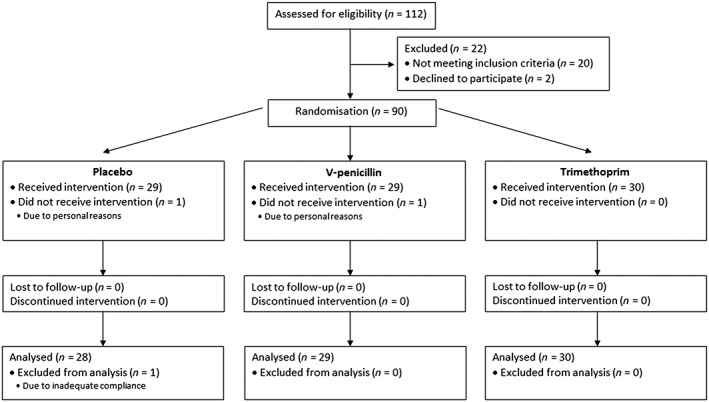
Participants flow for the PENTRIOX trial
Ninety participants were randomised to treatment. Two participants withdrew due to personal reasons, and one participant was excluded due to inadequate adherence.
Baseline clinical and biochemistry characteristics of the participants are presented in Table 2. The characteristics were within normal range and did not differ between groups.
Table 2.
Baseline clinical and biochemistry characteristics (PENTRIOX)
| Placebo | Penicillin V | Trimethoprim | P a | |
|---|---|---|---|---|
| n | 28 | 29 | 30 | |
| Age (years) | 24.4 (2.9) | 23.8 (3.0) | 25.2 (2.9) | NS |
| Weight (kg) | 78.5 (8.9) | 79.7 (8.0) | 81.3 (9.2) | NS |
| Height (m) | 1.84 (0.05) | 1.84 (0.05) | 1.87 (0.07) | NS |
| Body mass index (kg m 2 ) | 23.2 (2.1) | 23.6 (1.9) | 23.4 (2.3) | NS |
| Systolic blood pressure (mmHg) | 128.8 (7.0) | 129.7 (6.7) | 130.1 (8.2) | NS |
| Diastolic blood pressure (mmHg) | 77.0 (7.5) | 77.5 (7.5) | 77.0 (7.1) | NS |
| Pulse (beats min –1 ) | 66.2 (9.1) | 67.2 (12.8) | 68.2 (12.7) | NS |
| Total cholesterol (mmol l –1 ) | 4.4 (0.7) | 4.2 (0.6) | 4.2 (0.6) | NS |
| Serum creatinine (mmol l –1 ) | 78.7 (11.8) | 78.1 (12.3) | 79.3 (8.7) | NS |
| Ferritin (μmol l –1 ) | 126.1 (80.9) | 110.3 (74.9) | 120.9 (71.6) | NS |
| Iron (μmol l –1 ) | 18.8 (6.9) | 21.4 (8.8) | 21.7 (5.4) | NS |
| Transferrin saturation | 0.28 (0.12) | 0.32 (0.13) | 0.34 (0.09) | NS |
| Haemoglobin (mmol l –1 ) | 9.5 (0.5) | 9.4 (0.6) | 9.4 (0.5) | NS |
| Folate (mmol l –1 ) | 14.7 (7.4) | 13.9 (6.4) | 14.4 (5.8) | NS |
Values are presented as mean (standard deviation)
One‐way ANOVA; NS = not statistically significant
There was no significant difference in baseline urinary excretion of 8‐oxodG (P = 0.61) or 8‐oxoGuo (P = 0.93) between the groups.
According to trial participants, the overall adherence of urine samples was 94%. According to a control system based on phone text message from participants, adherence of medicine intake was 99%.
Seventeen participants reported a total of 23 AE. Ten were judged to be related to the medicine (AR), none were serious AE. Gastrointestinal discomfort was the most frequently observed event.
Primary endpoints
Data for primary endpoints (8‐oxodG and 8‐oxoGuo) are presented in Figure 3.
Figure 3.
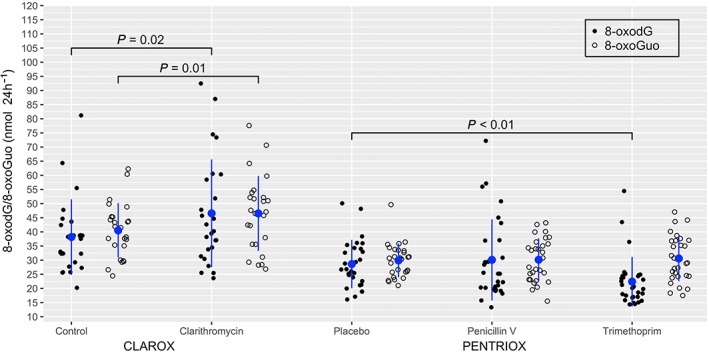
Values of urinary excretion of 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine (8‐oxodG; solid) and 8‐oxo‐7,8‐dihydroguanosine (8‐oxoGuo; open) for each participant after treatment. The data are further denoted as mean (blue circle) and standard deviation (blue line). Clarithromycin significantly increased urinary excretion of 8‐oxodG and 8‐oxoGuo compared to control. Trimethoprim significantly lowered urinary excretion of 8‐oxodG but not 8‐oxoGuo compared to placebo. Penicillin V had no significant effect on urinary excretion of 8‐oxodG or 8‐oxoGuo compared to placebo
Clarithromycin significantly induced DNA oxidation by increasing urinary excretion of 8‐oxodG with 22.0% (95% CI: 3.6–40.4%) compared to control (P = 0.02). Trimethoprim significantly lowered urinary excretion of 8‐oxodG by 21.7% (95% CI: 5.8–37.6%) compared to placebo (P < 0.01). There was no significant difference in urinary excretion of 8‐oxodG following penicillin V treatment compared to placebo (P = 0.63).
Clarithromycin significantly induced RNA oxidation by increasing urinary excretion of 8‐oxoGuo by 14.9% (95% CI: 3.7–26.1%) compared to control (P = 0.01). There was no statistically significant difference in urinary excretion of 8‐oxoGuo between placebo treatment and trimethoprim treatment (P = 0.71) or penicillin V treatment (P = 0.90).
Secondary endpoints
Data for secondary endpoint (MDA) are presented in Figure 4.
Figure 4.
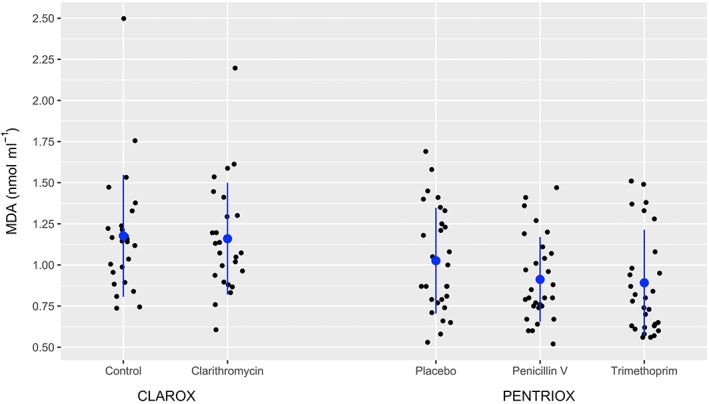
Values of plasma malondialdehyde (MDA; solid) for each participant after treatment. The data are further denoted as mean (blue circle) and standard deviation (blue line). There was no significant difference in MDA, when treatment with clarithromycin, penicillin V, and trimethoprim was compared to control/placebo
There was no statistically significance difference in MDA, when treatment with clarithromycin, penicillin V, and trimethoprim was compared to control/placebo (P > 0.05).
Discussion
This study is the first to demonstrate that 1 week treatment with clarithromycin increases oxidative nucleic acid modifications and 1 week treatment with trimethoprim lowers DNA oxidation in non‐infected humans. The bactericidal antibiotic penicillin V does not influence oxidative nucleic acid modifications.
Our study supports the finding that ROS production from antibiotic drugs is not restricted to bacteria but also takes place in mammalian cells 12, 13. This is not an intended effect when administering the drugs. Increased levels of oxidative modifications is linked to a number of different diseases 16, 17, 18, 19, and therefore increased oxidative modifications in relation to antibiotic treatment need confirmation and further exploration.
The biomarkers used to evaluate oxidative nucleic acid modifications are 8‐oxodG and 8‐oxoGuo. The biomarkers are both clinically relevant: 8‐oxodG has been identified as a biomarker associated with development of lung cancer in non‐smokers 22 and development of oestrogen receptor positive breast cancer 23; and 8‐oxoGuo is associated with overall mortality in patients with type 2 diabetes 20, 21. The clinical importance of RNA oxidation might be explained by the observation that RNA oxidation is able to induce cellular apoptosis in human cell lines 36 and translational errors in animal models 37, even though the qualitative effect remains unknown.
Neurodegenerative and cardiovascular diseases are also associated with elevated levels of oxidative modifications; however, the prognostic value of elevated oxidative modifications remain unknown in these diseases 24.
We did not investigate the effect of clarithromycin on glutathione – an important nonenzymatic antioxidant 14. The effect of clarithromycin on glutathione is an important subject for further research, since paracetamol depletes liver glutathione 38 and some patients have an inherited glucose‐6‐phosphate dehydrogenase deficiency that reduces the level of glutathione 39.
The observed effect of clarithromycin on urinary excretion of 8‐oxodG and 8‐oxoGuo compared to other lifestyle habits and diseases might seem small. Smoking increases urinary excretion of 8‐oxodG by 50% 40, untreated patients with hemochromatosis have a 2.5‐fold increased urinary excretion of 8‐oxoGuo 41, and patients with type 2 diabetes have a 2‐fold increased urinary excretion of 8‐oxoGuo in highest quartile compared to the lowest quartile associated with increased mortality 21. However, taking into account the short‐term exposure compared to long‐term lifestyle habits and chronic diseases the effect of clarithromycin treatment may still be relevant, particularly if certain organs are more affected than others.
There are several adverse reactions related to clarithromycin, where the mechanism is unknown. The CLARICOR trial demonstrated that 2 weeks’ treatment with clarithromycin 500 mg/day showed a significant increase in cardiovascular disease (i.e. cardiovascular mortality, myocardial infarction, unstable angina, cerebrovascular attack, or peripheral vascular disease) 3 years after treatment in patients with stable coronary heart diseases 25. However, the effect of clarithromycin on cardiovascular disease is not present, when participants were treated with statins at entry 42. The fact that our results illustrate how 1 week of clarithromycin treatment increases oxidative nucleic acid modifications, and that oxidative modifications are linked to cardiovascular diseases 19 make us hypothesize that the CLARICOR results 25 relate to increased oxidative nucleic acid modifications.
Even though statins lower calcium‐induced oxidative modifications 43, simvastatin did not reduce oxidative modifications in healthy humans 44. The limited and inconsistent literature exploring a possible antioxidant effect of statins are not sufficient to rule out whether the reversed effect of statins in the CLARICOR results could be explained by an antioxidant effect by the statins.
In addition, the present study demonstrates that trimethoprim lowers DNA oxidation but not RNA oxidation. To our knowledge, this is the first time a drug treatment has been found to lower the urinary excretion of 8‐oxodG. The result was unexpected, because methotrexate with same effect on folate as trimethoprim, although more pronounced, increases oxidative modifications 45. Further, the result is at variance with a previous in vitro study, where trimethoprim increased DNA damage measured by tail percentage in comet assay at dosage of 100 μg/mL in rainbow trout gonad‐2 cells, and had no effect on DNA damage in Chinese hamster ovary‐K1 cells 46. Our finding is worth noting, since increased levels of urinary excretion of 8‐oxodG have been proposed to be associated with the development of certain lung and breast cancer 22, 23.
Antibiotic drugs cause bacterial death. Therefore, it could be questioned if bacterial death increases the urinary excretion of 8‐oxodG and 8‐oxoGuo, thus acting as a possible confounder of the results in this study. However, previous studies have demonstrated that increased cell turnover does not increase urinary excretion of 8‐oxodG 47, 48, 49. This finding supports the accepted hypotheses that urinary excretion of 8‐oxodG is derived from repair of DNA oxidation 50, and we do not expect bacterial death to influence the result. To our knowledge, no studies have explored cell turnover as a possible confounder to urinary excretion of 8‐oxoGuo.
MDA is a biomarker of lipid peroxidation. ROS react with polyunsaturated acids and forms MDA as an end product 51. Surprisingly, none of the investigated antibiotics had any significant effect on the level of MDA. From this we conclude that: either the antibiotics do not induce lipid peroxidation measurable as MDA at the concentrations achieved in this study or oxidative modifications may be compartmentalized in the organism and within the cell. Although unbound MDA has been shown to be distributed relatively freely by passive diffusion despite its reactivity, most tissue and plasma MDA is considered to be protein bound 52, 53. However, in the present study, we measured total MDA.
Previous studies have shown that MDA is a valuable marker on a group base, in particular when measured by specific assays 27. Thus, the finding underlines that the term oxidative stress is relatively unspecific 54 and the notion that a number of biomarkers are necessary to understand the pathogenesis of different diseases that relates to oxidative modifications 51.
In order to achieve valid results, when measuring urinary excretion of 8‐oxodG and 8‐oxoGuo, the methodology is essential. Enzyme‐linked immunosorbent assay (ELISA) measurements are frequently used, because the method is inexpensive and easy to use. However, the method is limited by the lack of specificity (probably due to unspecific antibodies 55) compared to chromatography‐based techniques such liquid chromatography coupled with electrochemical detection (LC‐EC) or coupled with tandem mass‐spectrometry (LC‐MS/MS) 56. Thus, the mean values of the ELISA methods have turned out to be higher than the values produced by the chromatographic methods 57, 58 and the association between the methods are weak 59. The specificity of the ELISA method can be improved by a longer incubation at lower temperature i.e. 4°C, but there is still discrepancy between the methods 55. Liquid chromatography coupled with tandem mass‐spectrometry gives very high specificity and requires little sample preparation and is thus preferred to determine urinary excretion of 8‐oxodG and 8‐oxoGuo. The specificity of the method may be further improved by using ultra‐HLPC and a qualifier ion 56.
The same pattern is found in regards of methods to determine MDA. Colorimetric assays based on the TBA reaction or other conjugation reactions are frequently used because of their simplicity and low cost. However, the TBA and other colorimetric MDA assays lack specificity and has been shown to detect multiple specimens unrelated to MDA. In the literature, this has given rice to reference values that differ up to two orders of magnitude from the more reliable methods based on HPLC 27.
It has been widely accepted, that only bactericidal antibiotic induces ROS formation, hence contributing to the antibiotic effect in bacteria 2, 3, 4, 5, 6, 7, 8, 9, and this is also demonstrated in mammalian cells 12, 13. Our study does not investigate in vitro mechanisms, but the effect on whole body level. Therefore, the study might not detect changes in particular cells or a particular organ. However, it is surprisingly that clarithromycin, a bacteriostatic antibiotic, increases oxidative nucleic acid modifications which is not seen, when participants are treated with penicillin V, a bactericidal antibiotic. This finding is at variance with the well‐established hypothesis that only bactericidal antibiotic induces ROS formation – at least when the effect is measured on a whole‐body level in humans. Our study design does not allow an explanation of the underlying cellular mechanisms. However, we hypothesize that compartmentalization within the cell and organism of oxidative modifications could explain the inconsistence between the markers of lipid peroxidation and oxidative nucleic acid modifications. If this is the case, our results suggest that clarithromycin only increases intracellular oxidative modifications in a compartmentalized fashion.
Strengths and limitations
The strengths of the trials are the controlled and randomised trial designs, and the high level of adherence. There are limitations to our study. The trials have rather small sample sizes, and the open‐label design in the CLAROX trial could result in possible placebo effect and observer bias. However, the urine adherence collection is recorded with no difference between the groups, and the outcome of the trial is analysed without knowledge of the treatment.
Conclusion
In conclusion, we found that penicillin V has no effect, but clarithromycin increases whole body RNA and DNA oxidation in healthy humans. This finding raises the question whether increased oxidative modifications could explain some of the adverse reactions seen in relation to clarithromycin treatment.
To our surprise trimethoprim lowers DNA oxidation in healthy participants. To our knowledge, this is the first treatment found to lower the urinary excretion of 8‐oxodG, and merits verification and further investigation in future studies.
Competing Interests
There are no competing interests to declare.
We thank senior laboratory technician Lis Kjær Hansen and laboratory technicians Katja L. Christensen, Tanja M. Nielsen and Mads Sabroe for performing the urine analyses. Furthermore, we thank Annie B. Kristensen and Joan E. Frandsen for performing the plasma analyses.
This research was supported by a grant from The Research Council of the Capital Region of Copenhagen and internal funds from the Department of Clinical Pharmacology and Laboratory of Clinical Pharmacology, Copenhagen University Hospital Bispebjerg Frederiksberg.
V.C. has received the Faculty PhD Scholarship from the Faculty of Health and Medical Sciences, University of Copenhagen.
Contributors
E.L., H.P., V.C. and L.K.K. wrote the article; H.P., M.T.P, S.P. and L.K.H. designed the research; H.P., M.T.P, S.P., L.K.H., J.A., E.J.S., K.B., M.P., A.W., T.H. and J.L performed the research; E.L., H.P., V.C., L.K.K. and C.T.P. analysed data.
Supporting information
Information S1 Trial design
Information S2 Exclusion criteria
Information S3 Representative high‐performance liquid chromatogram
Larsen, E. L. , Cejvanovic, V. , Kjær, L. K. , Pedersen, M. T. , Popik, S. D. , Hansen, L. K. , Andersen, J. T. , Jimenez‐Solem, E. , Broedbaek, K. , Petersen, M. , Weimann, A. , Henriksen, T. , Lykkesfeldt, J. , Torp‐Pedersen, C. , and Poulsen, H. E. (2017) Clarithromycin, trimethoprim, and penicillin and oxidative nucleic acid modifications in humans: randomised, controlled trials. Br J Clin Pharmacol, 83: 1643–1653. doi: 10.1111/bcp.13261.
References
- 1. Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram‐positive bacterial infections. Clin Infect Dis 2004; 38: 864–870. [DOI] [PubMed] [Google Scholar]
- 2. Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007; 130: 797–810. [DOI] [PubMed] [Google Scholar]
- 3. Wang X, Zhao X. Contribution of oxidative damage to antimicrobial lethality. Antimicrob Agents Chemother 2009; 53: 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu Y, Liu X, Qu Y, Wang X, Li L, Zhao X. Inhibitors of reactive oxygen species accumulation delay and/or reduce the lethality of several antistaphylococcal agents. Antimicrob Agents Chemother 2012; 56: 6048–6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grant SS, Kaufmann BB, Chand NS, Haseley N, Hung DT. Eradication of bacterial persisters with antibiotic‐generated hydroxyl radicals. Proc Natl Acad Sci U S A 2012; 109: 12147–12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shatalin K, Shatalina E, Mironov A, Nudler E. H2S: a universal defense against antibiotics in bacteria. Science 2012; 334: 1215–1220. [DOI] [PubMed] [Google Scholar]
- 7. Wang X, Zhao X, Malik M, Drlica K. Contribution of reactive oxygen species to pathways of quinolone‐mediated bacterial cell death. J Antimicrob Chemother 2010; 65: 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science 2012; 336: 315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dwyer DJ, Kohanski MA, Hayete B, Collins JJ. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli . Mol Syst Biol 2007; 3: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keren I, Yanxia W, Inocencio J, Mulcahy LR, Lewis K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science 2013; 339: 1213–1216. [DOI] [PubMed] [Google Scholar]
- 11. Liu Y, Imlay JA. Cell death from antibiotics without the involvement of reactive oxygen species. Science 2013; 339: 1210–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalghatgi S, Spina CS, Costello JC, Liesa M, Morones‐Ramirez JR, Slomovic S, et al. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in mammalian cells. Sci Transl Med 2013; 5: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kohanski MA, Tharakan A, Lane AP, Ramanathan M. Bactericidal antibiotics promote reactive oxygen species formation and inflammation in human sinonasal epithelial cells. Int Forum Allergy Rhinol 2016; 6: 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jacob KD, Noren Hooten N, Trzeciak AR, Evans MK. Markers of oxidant stress that are clinically relevant in aging and age‐related disease. Mech Ageing Dev 2013; 134: 139–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Broedbaek K, Weimann A, Stovgaard ES, Poulsen HE. Urinary 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine as a biomarker in type 2 diabetes. Free Radic Biol Med 2011; 51: 1473–1479. [DOI] [PubMed] [Google Scholar]
- 16. Butterfield DA, Gnjec A, Poon HF, Castegna A, Pierce WM, Klein JB, et al. Redox proteomics identification of oxidatively modified brain proteins in inherited Alzheimer's disease: an initial assessment. J Alzheimers Dis 2006; 10: 391–397. [DOI] [PubMed] [Google Scholar]
- 17. Wood‐Kaczmar A, Gandhi S, Wood NW. Understanding the molecular causes of Parkinson's disease. Trends Mol Med 2006; 12: 521–528. [DOI] [PubMed] [Google Scholar]
- 18. Verdile G, Fuller SJ, Martins RN. The role of type 2 diabetes in neurodegeneration. Neurobiol Dis 2015; 84: 22–38. [DOI] [PubMed] [Google Scholar]
- 19. Santilli F, D'Ardes D, Davì G. Oxidative stress in chronic vascular disease: from prediction to prevention. Vascul Pharmacol 2015; 74: 23–37. [DOI] [PubMed] [Google Scholar]
- 20. Broedbaek K, Siersma V, Henriksen T, Weimann A, Petersen M, Andersen JT, et al. Urinary markers of nucleic acid oxidation and long‐term mortality of newly diagnosed type 2 diabetic patients. Diabetes Care 2011; 34: 2594–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Broedbaek K, Siersma V, Henriksen T, Weimann A, Petersen M, Andersen JT, et al. Association between urinary markers of nucleic acid oxidation and mortality in type 2 diabetes: a population‐based cohort study. Diabetes Care 2013; 36: 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loft S, Svoboda P, Kasai H, Tjønneland A, Vogel U, Møller P, et al. Prospective study of 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine excretion and the risk of lung cancer. Carcinogenesis 2006; 27: 1245–1250. [DOI] [PubMed] [Google Scholar]
- 23. Loft S, Olsen A, Møller P, Poulsen HE, Tjønneland A. Association between 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine excretion and risk of postmenopausal breast cancer: nested case–control study. Cancer Epidemiol Biomarkers Prev 2013; 22: 1289–1296. [DOI] [PubMed] [Google Scholar]
- 24. Poulsen HE, Specht E, Broedbaek K, Henriksen T, Ellervik C, Mandrup‐Poulsen T, et al. RNA modifications by oxidation: A novel disease mechanism? Free Radic Biol Med 2012; 52: 1353–1361. [DOI] [PubMed] [Google Scholar]
- 25. Jespersen CM, Als‐Nielsen B, Damgaard M, Hansen JF, Hansen S, Helø OH, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ 2006; 332: 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poulsen HE, Nadal LL, Broedbaek K, Nielsen PE, Weimann A. Detection and interpretation of 8‐oxodG and 8‐oxoGua in urine, plasma and cerebrospinal fluid. Biochim Biophys Acta ‐ Gen Subj 1840; 2014: 801–808. [DOI] [PubMed] [Google Scholar]
- 27. Lykkesfeldt J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin Chim Acta 2007; 380: 50–58. [DOI] [PubMed] [Google Scholar]
- 28. Klacid Uno® (BGP Products; Maidenhead, United Kingdom) http: //pro.medicin.dk/Medicin/Praeparater/1502 (accessed June 8, 2016).
- 29. Pancillin® (Sandoz; Holzkirchen, Germany) http: //pro.medicin.dk/Medicin/Praeparater/1759 (accessed June 8, 2016).
- 30. Trimopan® (Orion Pharma; Espoo, Finland) http: //pro.medicin.dk/Medicin/Praeparater/467 (accessed June 8, 2016).
- 31. European‐Commision . Good Manufacturing Practice. Medicinal Products for Human and Vetrinary use. Annex 13. Investigational Medicinal Prodcts 2010: 4. [Google Scholar]
- 32. ICH Harmonised Tripartite Guideline . Guideline for Good Clinical Practice E6(R1). ICH Harmon Tripart Guidel 1996. [Google Scholar]
- 33. Henriksen T, Hillestrøm PR, Poulsen HE, Weimann A. Automated method for the direct analysis of 8‐oxo‐guanosine and 8‐oxo‐2′‐deoxyguanosine in human urine using ultraperformance liquid chromatography and tandem mass spectrometry. Free Radic Biol Med 2009; 47: 629–635. [DOI] [PubMed] [Google Scholar]
- 34. Lykkesfeldt J. Determination of malondialdehyde as dithiobarbituric acid adduct in biological samples by HPLC with fluorescence detection: comparison with ultraviolet‐visible spectrophotometry. Clin Chem 2001; 47: 1725–1727. [PubMed] [Google Scholar]
- 35. Core Team R (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at http: //www.r‐project.org/ (last accessed 8 June 2016).
- 36. Wang JX, Gao J, Ding SL, Wang K, Jiao JQ, Wang Y, et al. Oxidative modification of miR‐184 enables it to target Bcl‐xL and Bcl‐w. Mol Cell 2015; 59: 50–61. [DOI] [PubMed] [Google Scholar]
- 37. Tanaka M, Chock PB, Stadtman ER. Oxidized messenger RNA induces translation errors. Proc Natl Acad Sci 2006; 104: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao L, Pickering G. Paracetamol metabolism and related genetic differences. Drug Metab Rev 2011; 43: 41–52. [DOI] [PubMed] [Google Scholar]
- 39. Luzzatto L, Nannelli C, Notaro R. Glucose‐6‐phosphate dehydrogenase deficiency. Hematol Oncol Clin North Am 2016; 30: 373–393. [DOI] [PubMed] [Google Scholar]
- 40. Loft S, Vistisen K, Ewertz M, Tjønneland A, Overvad K, Poulsen HE. Oxidative DNA damage estimated by 8‐hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis 1992; 13: 2241–2247. [DOI] [PubMed] [Google Scholar]
- 41. Broedbaek K, Poulsen HE, Weimann A, Kom GD, Schwedhelm E, Nielsen P, et al. Urinary excretion of biomarkers of oxidatively damaged DNA and RNA in hereditary hemochromatosis. Free Radic Biol Med 2009; 47: 1230–1233. [DOI] [PubMed] [Google Scholar]
- 42. Winkel P, Hilden J, Hansen JF, Kastrup J, Kolmos HJ, Kjøller E, et al. Clarithromycin for stable coronary heart disease increases all‐cause and cardiovascular mortality and cerebrovascular morbidity over 10 years in the CLARICOR randomised, blinded clinical trial. Int J Cardiol 2015; 182: 459–465. [DOI] [PubMed] [Google Scholar]
- 43. Parihar A, Parihar MS, Zenebe WJ, Ghafourifar P. Statins lower calcium‐induced oxidative stress in isolated mitochondria. 2016. [DOI] [PubMed]
- 44. Rasmussen ST, Andersen JT, Nielsen TK, Cejvanovic V, Petersen KM, Henriksen T, et al. Simvastatin and oxidative stress in humans: a randomized, double‐blinded, placebo‐controlled clinical trial. Redox Biol 2016; 9: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tabassum H, Parvez S, Pasha ST, Banerjee BD, Raisuddin S. Protective effect of lipoic acid against methotrexate‐induced oxidative stress in liver mitochondria. Food Chem Toxicol 2010; 48: 1973–1979. [DOI] [PubMed] [Google Scholar]
- 46. Papis E, Davies SJ, Jha AN. Relative sensitivity of fish and mammalian cells to the antibiotic, trimethoprim: cytotoxic and genotoxic responses as determined by neutral red retention, Comet and micronucleus assays. Ecotoxicology 2011; 20: 208–217. [DOI] [PubMed] [Google Scholar]
- 47. Erhola M, Toyokuni S, Okada K, Tanaka T, Hiai H, Ochi H, et al. Biomarker evidence of DNA oxidation in lung cancer patients: association of urinary 8‐hydroxy‐2′‐deoxyguanosine excretion with radiotherapy, chemotherapy, and response to treatment. FEBS Lett 1997; 409: 287–291. [DOI] [PubMed] [Google Scholar]
- 48. Faure H, Mousseau M, Cadet J, Guimier C, Tripier M, Hida H, et al. Urine 8‐oxo‐7, 8‐dihydro‐2′‐deoxyguanosine vs. 5–(hydroxymethyl) uracil as DNA oxidation marker in adriamycin‐treated patients. Free Radic Res 1998; 28: 377–382. [DOI] [PubMed] [Google Scholar]
- 49. Siomek A, Tujakowski J, Gackowski D, Rozalski R, Foksinski M, Dziaman T, et al. Severe oxidatively damaged DNA after cisplatin treatment of cancer patients. Int J Cancer 2006; 119: 2228–2230. [DOI] [PubMed] [Google Scholar]
- 50. Evans MD, Saparbaev M, Cooke MS. DNA repair and the origins of urinary oxidized 2′‐ deoxyribonucleosides. Mutagenesis 2010; 25: 433–442. [DOI] [PubMed] [Google Scholar]
- 51. Frijhoff J, Winyard PG, Zarkovic N, Davies SS, Stocker R, Cheng D, et al. Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal 2015; 23: 1144–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marnett LJ, Buck J, Tuttle MA, Basu AK, Bull AW. Distribution and oxidation of malondialdehyde in mice. Prostaglandins 1985; 30: 241–254. [DOI] [PubMed] [Google Scholar]
- 53. Draper HH, Squires EJ, Mahmoodi H, Wu J, Agarwal S, Hadley M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic Biol Med 1993; 15: 353–363. [DOI] [PubMed] [Google Scholar]
- 54. Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol 2015; 4: 180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Evans MD, Singh R, Mistry V, Sandhu K, Farmer PB, Cooke MS. Analysis of urinary 8‐oxo‐7,8‐dihydro‐purine‐2′‐deoxyribonucleosides by LC‐MS/MS and improved ELISA. Free Radic Res 2008; 42: 831–840. [DOI] [PubMed] [Google Scholar]
- 56. Weimann A, Broedbaek K, Henriksen T, Stovgaard ES, Poulsen HE. Assays for urinary biomarkers of oxidatively damaged nucleic acids. Free Radic Res 2012; 46: 531–540. [DOI] [PubMed] [Google Scholar]
- 57. Yoshida R, Ogawa Y, Kasai H. Urinary 8‐oxo‐7,8‐dihydro‐2 ’‐deoxyguanosine values measured by an ELISA correlated well with measurements by high‐performance liquid chromatography with electrochemical detection. Cancer Epidemiol Biomarkers Prev 2002; 11: 1076–1081. [PubMed] [Google Scholar]
- 58. Shimoi K, Kasai H, Yokota N, Toyokuni S, Kinae N. Comparison between high‐performance liquid chromatography and enzyme‐linked immunosorbent assay for the determination of 8‐hydroxy‐2′‐deoxyguanosine in human urine. Cancer Epidemiol Biomarkers Prev 2002; 11: 767–770. [PubMed] [Google Scholar]
- 59. Cooke MS, Barregard L, Mistry V, Potdar N, Rozalski R, Gackowski D, et al. Interlaboratory comparison of methodologies for the measurement of urinary 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine. Biomarkers 2009; 14: 103–110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Information S1 Trial design
Information S2 Exclusion criteria
Information S3 Representative high‐performance liquid chromatogram


