Abstract
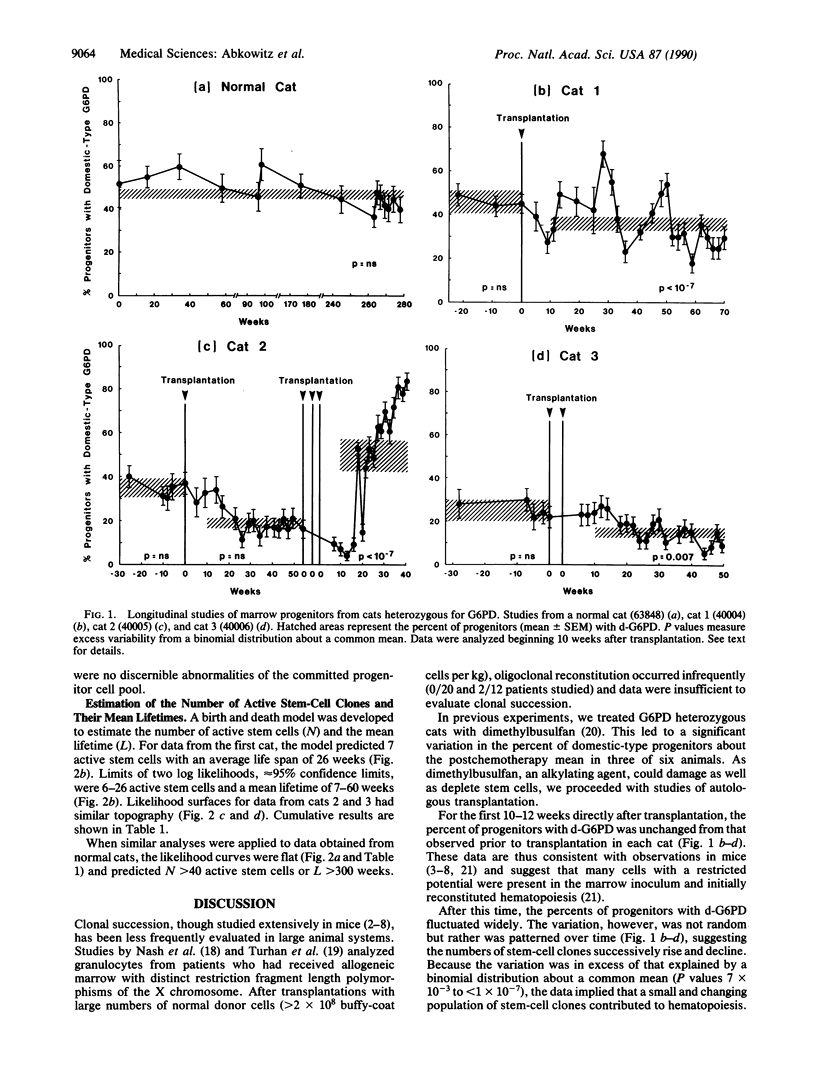
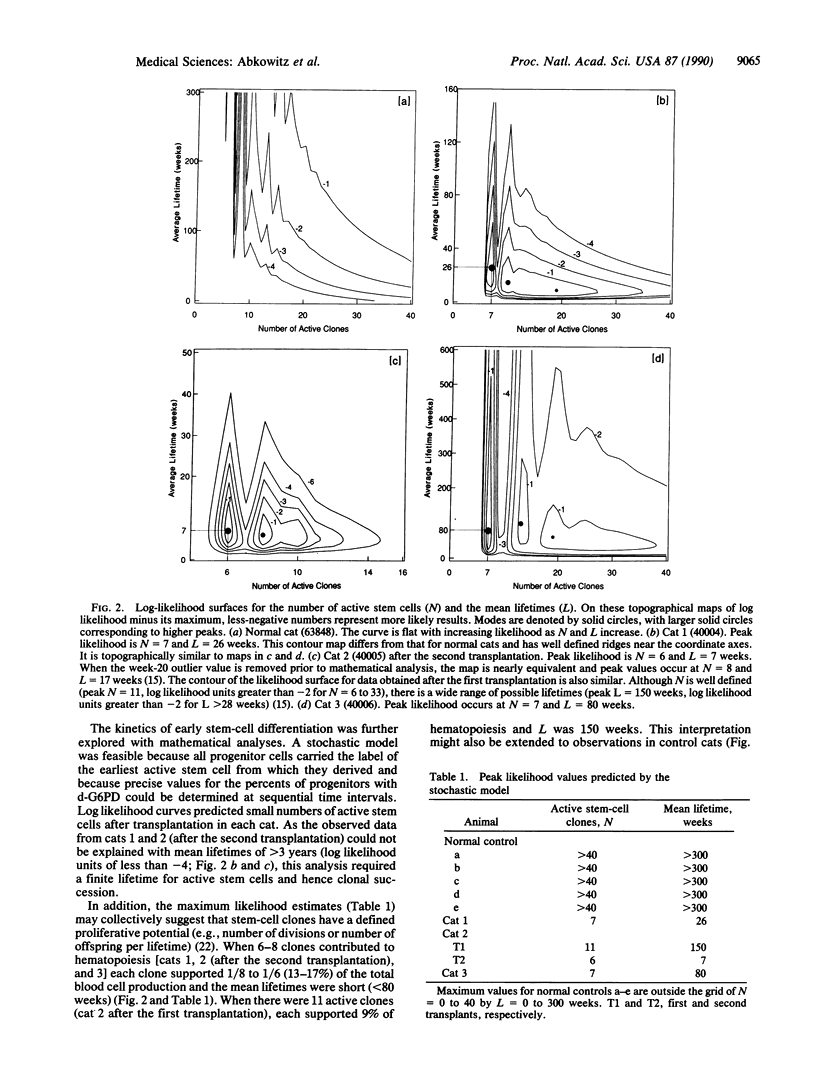
To test if hematopoiesis can be maintained by the sequential activation of stem-cell clones, we performed autologous marrow transplantations with limited numbers of cells in cats heterozygous for the X chromosome-linked enzyme glucose-6-phosphate dehydrogenase (G6PD) and observed the G6PD phenotypes of erythroid and granulocyte/macrophage progenitors over time. The animals were the female offspring of Geoffroy male and domestic female cats. In repeated studies of marrow from control animals (n = 5) or experimental animals prior to transplantation (n = 3), the percent of progenitors with domestic-type G6PD did not vary. After transplantation, the peripheral blood counts, marrow morphologies, frequencies of progenitors, and progenitor cell cycle kinetics returned to normal. However, abrupt and significant fluctuations were seen in the G6PD type of progenitors from each cat during the 1-1.5 years of observation. These data cannot be explained if there were either a large or constant population of active stem cells and thus imply, in a large-animal system, that hematopoiesis was maintained through clonal succession. A stochastic model was developed to estimate the numbers of active clones and their mean lifetimes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abkowitz J. L., Holly R. D., Adamson J. W. Retrovirus-induced feline pure red cell aplasia: the kinetics of erythroid marrow failure. J Cell Physiol. 1987 Sep;132(3):571–577. doi: 10.1002/jcp.1041320322. [DOI] [PubMed] [Google Scholar]
- Abkowitz J. L., Holly R. D., Segal G. M., Adamson J. W. Multilineage, non-species specific hematopoietic growth factor(s) elaborated by a feline fibroblast cell line: enhancement by virus infection. J Cell Physiol. 1986 Apr;127(1):189–196. doi: 10.1002/jcp.1041270123. [DOI] [PubMed] [Google Scholar]
- Abkowitz J. L., Ott R. L., Nakamura J. M., Steinmann L., Fialkow P. J., Adamson J. W. Feline glucose-6-phosphate dehydrogenase cellular mosaicism. Application to the study of retrovirus-induced pure red cell aplasia. J Clin Invest. 1985 Jan;75(1):133–140. doi: 10.1172/JCI111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abkowitz J. L., Ott R. M., Holly R. D., Adamson J. W. Clonal evolution following chemotherapy-induced stem cell depletion in cats heterozygous for glucose-6-phosphate dehydrogenase. Blood. 1988 Jun;71(6):1687–1692. [PubMed] [Google Scholar]
- Broudy V. C., Tait J. F., Powell J. S. Recombinant human erythropoietin: purification and analysis of carbohydrate linkage. Arch Biochem Biophys. 1988 Sep;265(2):329–336. doi: 10.1016/0003-9861(88)90135-x. [DOI] [PubMed] [Google Scholar]
- Buescher E. S., Alling D. W., Gallin J. I. Use of an X-linked human neutrophil marker to estimate timing of lyonization and size of the dividing stem cell pool. J Clin Invest. 1985 Oct;76(4):1581–1584. doi: 10.1172/JCI112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel B., Hawley R., Covarrubias L., Hawley T., Mintz B. Clonal contributions of small numbers of retrovirally marked hematopoietic stem cells engrafted in unirradiated neonatal W/Wv mice. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4564–4568. doi: 10.1073/pnas.86.12.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumenil D., Jacquemin-Sablon H., Neel H., Frindel E., Dautry F. Mock retroviral infection alters the developmental potential of murine bone marrow stem cells. Mol Cell Biol. 1989 Oct;9(10):4541–4544. doi: 10.1128/mcb.9.10.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E., Astle C. M., Lerner C. Number and continuous proliferative pattern of transplanted primitive immunohematopoietic stem cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):822–826. doi: 10.1073/pnas.85.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E., Astle C. M., Stone M. Numbers and functions of transplantable primitive immunohematopoietic stem cells. Effects of age. J Immunol. 1989 Jun 1;142(11):3833–3840. [PubMed] [Google Scholar]
- Harrison D. E., Lerner C., Hoppe P. C., Carlson G. A., Alling D. Large numbers of primitive stem cells are active simultaneously in aggregated embryo chimeric mice. Blood. 1987 Mar;69(3):773–777. [PubMed] [Google Scholar]
- Jones R. J., Celano P., Sharkis S. J., Sensenbrenner L. L. Two phases of engraftment established by serial bone marrow transplantation in mice. Blood. 1989 Feb;73(2):397–401. [PubMed] [Google Scholar]
- Jordan C. T., Lemischka I. R. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990 Feb;4(2):220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- KAY H. E. HOW MANY CELL-GENERATIONS? Lancet. 1965 Aug 28;2(7409):418–419. doi: 10.1016/s0140-6736(65)90763-4. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Mauch P., Hellman S. Loss of hematopoietic stem cell self-renewal after bone marrow transplantation. Blood. 1989 Aug 1;74(2):872–875. [PubMed] [Google Scholar]
- Micklem H. S., Lennon J. E., Ansell J. D., Gray R. A. Numbers and dispersion of repopulating hematopoietic cell clones in radiation chimeras as functions of injected cell dose. Exp Hematol. 1987 Mar;15(3):251–257. [PubMed] [Google Scholar]
- Nakahata T., Gross A. J., Ogawa M. A stochastic model of self-renewal and commitment to differentiation of the primitive hemopoietic stem cells in culture. J Cell Physiol. 1982 Dec;113(3):455–458. doi: 10.1002/jcp.1041130314. [DOI] [PubMed] [Google Scholar]
- Nakano T., Waki N., Asai H., Kitamura Y. Long-term monoclonal reconstitution of erythropoiesis in genetically anemic W/Wv mice by injection of 5-fluorouracil-treated bone marrow cells of Pgk-1b/Pgk-1a mice. Blood. 1987 Dec;70(6):1758–1763. [PubMed] [Google Scholar]
- Nash R., Storb R., Neiman P. Polyclonal reconstitution of human marrow after allogeneic bone marrow transplantation. Blood. 1988 Dec;72(6):2031–2037. [PubMed] [Google Scholar]
- Snodgrass R., Keller G. Clonal fluctuation within the haematopoietic system of mice reconstituted with retrovirus-infected stem cells. EMBO J. 1987 Dec 20;6(13):3955–3960. doi: 10.1002/j.1460-2075.1987.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILL J. E., MCCULLOCH E. A., SIMINOVITCH L. A STOCHASTIC MODEL OF STEM CELL PROLIFERATION, BASED ON THE GROWTH OF SPLEEN COLONY-FORMING CELLS. Proc Natl Acad Sci U S A. 1964 Jan;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turhan A. G., Humphries R. K., Phillips G. L., Eaves A. C., Eaves C. J. Clonal hematopoiesis demonstrated by X-linked DNA polymorphisms after allogeneic bone marrow transplantation. N Engl J Med. 1989 Jun 22;320(25):1655–1661. doi: 10.1056/NEJM198906223202504. [DOI] [PubMed] [Google Scholar]


