Abstract
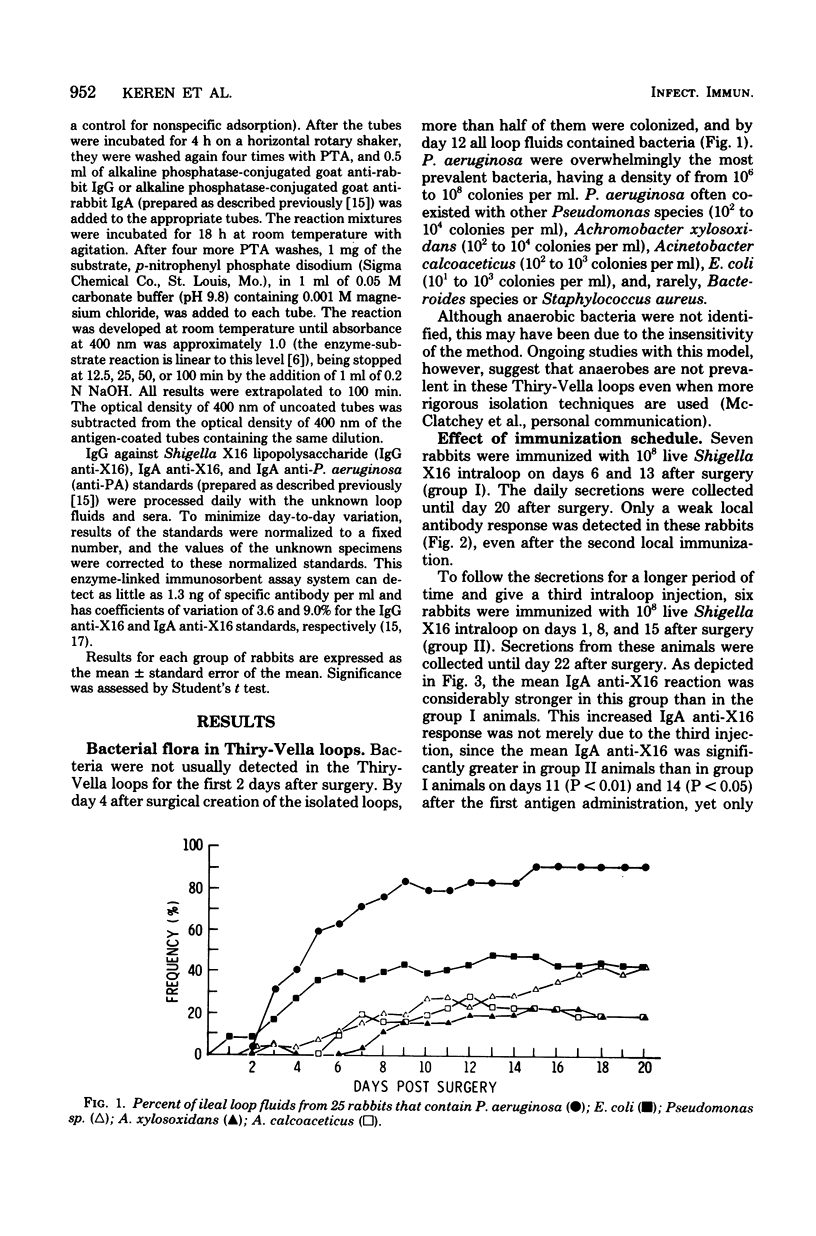
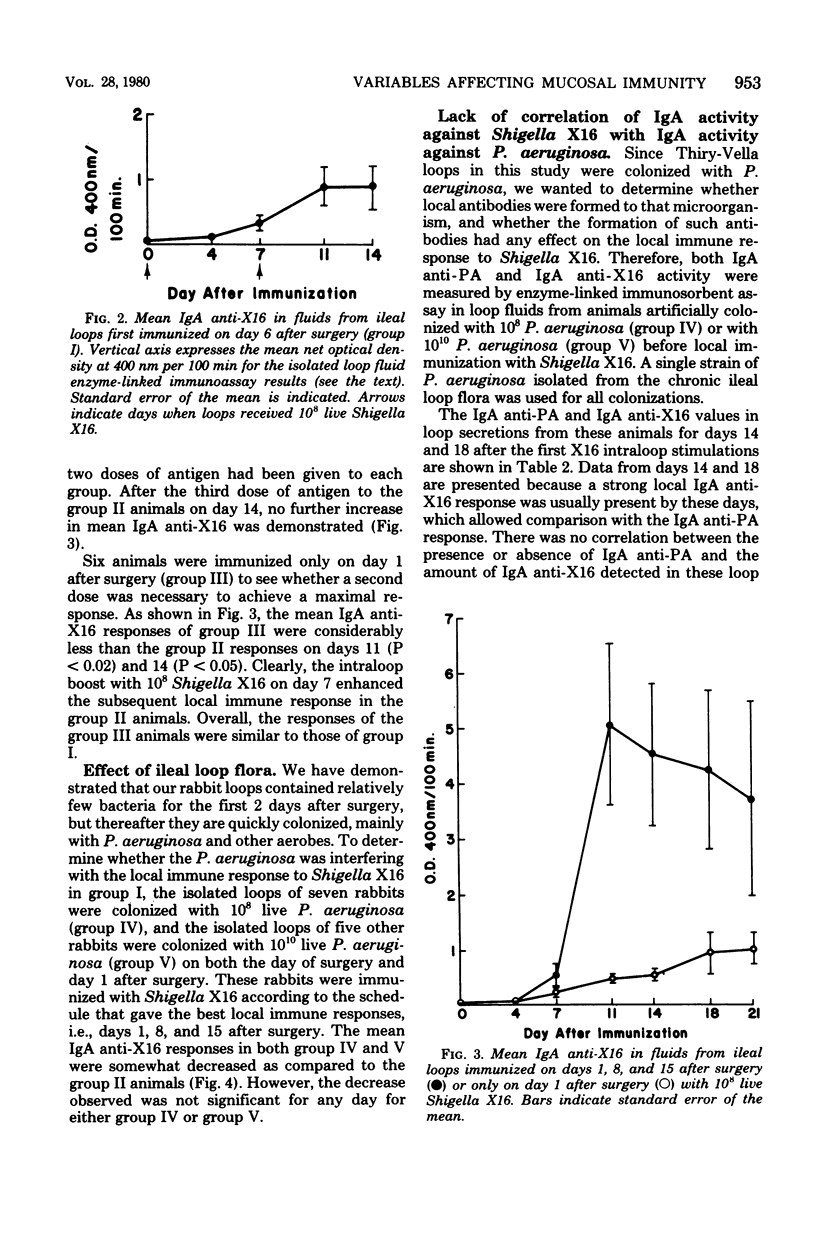
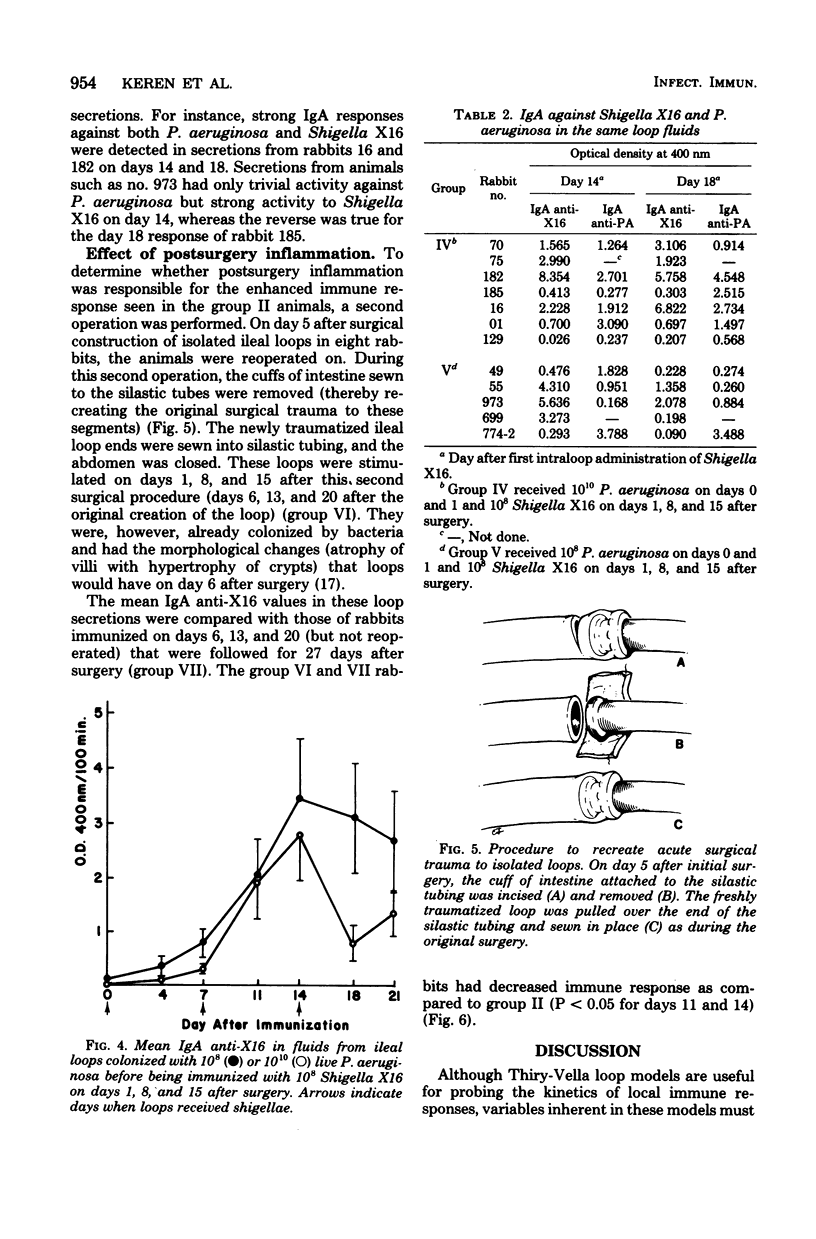
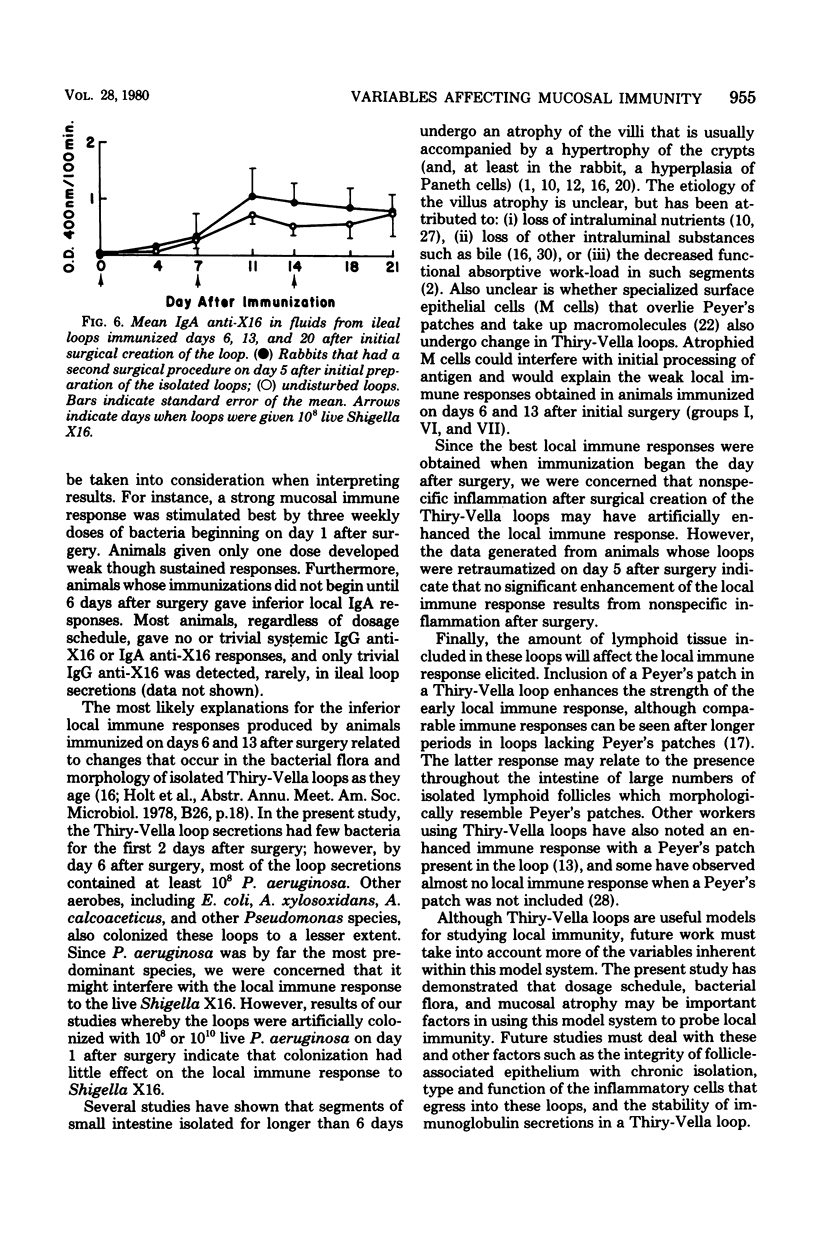
Several variables inherent in chronically isolated ileal (Thiry-Vella) loops in rabbits were studied for their effect on the local immune response of the intestine to live, locally invasive bacteria (Shigella X16). A much more vigorous local immunoglobulin A response to Shigella X16 was elicited when rabbits were immunized in their Thiry-Vella loops shortly after surgical creation of the loop than if a week were allowed to pass before they were immunized. Three major differences existed in Thiry-Vella loops on the day after surgery and a week later: (i) their microbial flora, (ii) nonspecific acute inflammation due to the surgery itself, and (iii) the histological appearance of the intestine. On day 1 after surgical creation of the Thiry-Vella loop, there were few bacteria in the loop, and the histology was that of normal small bowel except for mild acute inflammation due to the surgery. By day 6 after surgery, all loops contained large numbers of Pseudomonas aeruginosa and other aerobes, an atrophy of intestinal epithelium occurred, and the acute inflammation due to surgical trauma had subsided. By artificially colonizing Thiry-Vella loops with 108 or 1010 live P. aeruginosa on the day of surgery, we found that the presence of these bacteria alone did not greatly diminish local immune responses to live Shigella. Furthermore, when the acute inflammation due to surgical trauma was recreated in loops 6 days old, no enhancement of the immune response was seen as compared to nontraumatized 6-day-old Thiry-Vella loops. The difference between immunization soon after surgery and a week later related to changes that occur in the loop itself with increased isolation. Finally, multiple immunizations of Thiry-Vella loops resulted in a more vigorous local immunoglobulin A response than a single immunization. These studies demonstrated that Thiry-Vella loop models can be useful in studying the kinetics of local immune responses by the intestine only if careful attention is paid to key variables inherent in the Thiry-Vella loop models themselves.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann G. G., Leblond C. P. Factors influencing villus size in the small intestine of adult rats as revealed by transposition of intestinal segments. Am J Anat. 1970 Jan;127(1):15–36. doi: 10.1002/aja.1001270104. [DOI] [PubMed] [Google Scholar]
- Clarke R. M. "Luminal nutrition" versus "functional work-load" as controllers of mucosal morphology and epithelial replacement in the rat small intestine. Digestion. 1977;15(5):411–424. doi: 10.1159/000198029. [DOI] [PubMed] [Google Scholar]
- Crabbé P. A., Nash D. R., Bazin H., Eyssen D. V., Heremans J. F. Antibodies of the IgA type in intestinal plasma cells of germfree mice after oral or parenteral immunization with ferritin. J Exp Med. 1969 Oct 1;130(4):723–744. doi: 10.1084/jem.130.4.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971 Jul 1;134(1):188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- FORMAL S. B., LABREC E. H., KENT T. H., FALKOW S. ABORTIVE INTESTINAL INFECTION WITH AN ESCHERICHIA COLI-SHIGELLA FLEXNERI HYBRID STRAIN. J Bacteriol. 1965 May;89:1374–1382. doi: 10.1128/jb.89.5.1374-1382.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Kent T. H., Austin S., Labrec E. H. Fluorescent-antibody and histological study of vaccinated and control monkeys challenged with Shigella flexneri. J Bacteriol. 1966 Jun;91(6):2368–2376. doi: 10.1128/jb.91.6.2368-2376.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fubara E. S., Freter R. Protection against enteric bacterial infection by secretory IgA antibodies. J Immunol. 1973 Aug;111(2):395–403. [PubMed] [Google Scholar]
- Gleeson M. H., Cullen J., Dowling R. H. Intestinal structure and function after small bowel by-pass in the rat. Clin Sci. 1972 Dec;43(6):731–742. doi: 10.1042/cs0430731. [DOI] [PubMed] [Google Scholar]
- Hamilton S. R., Yardley J. H., Brown G. D. Suppression of local intestinal immunoglobulin A immune response to cholera toxin by subcutaneous administration of cholera toxoids. Infect Immun. 1979 May;24(2):422–426. doi: 10.1128/iai.24.2.422-426.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietanen E., Hänninen O. Effect of chyme on mucosal enzyme levels in small intestine of the rat. Metabolism. 1972 Nov;21(11):991–1000. doi: 10.1016/0026-0495(72)90029-7. [DOI] [PubMed] [Google Scholar]
- Husband A. J., Gowans J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978 Nov 1;148(5):1146–1160. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband A. J., Lascelles A. K. The origin of antibody in intestinal secretion of sheep. Aust J Exp Biol Med Sci. 1974 Oct;52(5):791–799. doi: 10.1038/icb.1974.78. [DOI] [PubMed] [Google Scholar]
- Keren D. F., Elliott H. L., Brown G. D., Yardley J. H. Atrophy of villi with hypertrophy and hyperplasia of Paneth cells in isolated (thiry-Vella) ileal loops in rabbits. Light-microscopic studies. Gastroenterology. 1975 Jan;68(1):83–93. [PubMed] [Google Scholar]
- Keren D. F. Enzyme-linked immunosorbent assay for immunoglobulin G and immunoglobulin A antibodies to Shigella flexneri antigens. Infect Immun. 1979 May;24(2):441–448. doi: 10.1128/iai.24.2.441-448.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren D. F., Holt P. S., Collins H. H., Gemski P., Formal S. B. The role of Peyer's patches in the local immune response of rabbit ileum to live bacteria. J Immunol. 1978 Jun;120(6):1892–1896. [PubMed] [Google Scholar]
- Mebus C. A., Torres-Medina A., Twiehaus M. J., Bass E. P. Immune response to orally administered calf reovirus-like agent and coronavirus vaccine. Dev Biol Stand. 1976;33:396–403. [PubMed] [Google Scholar]
- Mel D. M., Terzin A. L., Vuksić L. Studies on vaccination against bacillary dysentery. 3. Effective oral immunization against Shigella flexneri 2a in a field trial. Bull World Health Organ. 1965;32(5):647–655. [PMC free article] [PubMed] [Google Scholar]
- Menge H., Bloch R., Schaumlöffel E., Riecken E. O. Transportstudien, morphologische, morphometrische und histochemische Untersuchungen zum Verhalten der Dünndarmschleimhaut im operativ ausgeschalteten Jejunalabschnitt der Ratte. Z Gesamte Exp Med. 1970;153(1):74–90. [PubMed] [Google Scholar]
- Michalek S. M., McGhee J. R., Babb J. L. Effective immunity to dental caries: dose-dependent studies of secretory immunity by oral administration of Streptococcus mutans to rats. Infect Immun. 1978 Jan;19(1):217–224. doi: 10.1128/iai.19.1.217-224.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. L. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology. 1977 Mar;72(3):440–451. [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J Exp Med. 1978 Jul 1;148(1):195–206. doi: 10.1084/jem.148.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter P., Kenworthy R., Noakes D. E., Allen W. D. Intestinal antibody secretion in the young pig in response to oral immunization with Escherichia coli. Immunology. 1974 Nov;27(5):841–853. [PMC free article] [PubMed] [Google Scholar]
- Rijke R. P., Plaisier H. M., de Ruiter H., Galjaard H. Influence of experimental bypass on cellular kinetics and maturation of small intestinal epithelium in the rat. Gastroenterology. 1977 May;72(5 Pt 1):896–901. [PubMed] [Google Scholar]
- Sack R. B., Johnson J., Pierce N. F., Keren D. F., Yardley J. H. Challenge of dogs with live enterotoxigenic Escherichia coli and effects of repeated challenges on fluid secretion in jejunal Thiry-Vella loops. J Infect Dis. 1976 Jul;134(1):15–24. doi: 10.1093/infdis/134.1.15. [DOI] [PubMed] [Google Scholar]
- Weser E., Heller R., Tawil T. Stimulation of mucosal growth in the rat ileum by bile and pancreatic secretions after jejunal resection. Gastroenterology. 1977 Sep;73(3):524–529. [PubMed] [Google Scholar]
- Yardley J. H., Keren D. F., Hamilton S. R., Brown G. D. Local (immunoglobulin A) immune response by the intestine to cholera toxin and its partial suppression with combined systemic and intra-intestinal immunization. Infect Immun. 1978 Feb;19(2):589–597. doi: 10.1128/iai.19.2.589-597.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


