Abstract
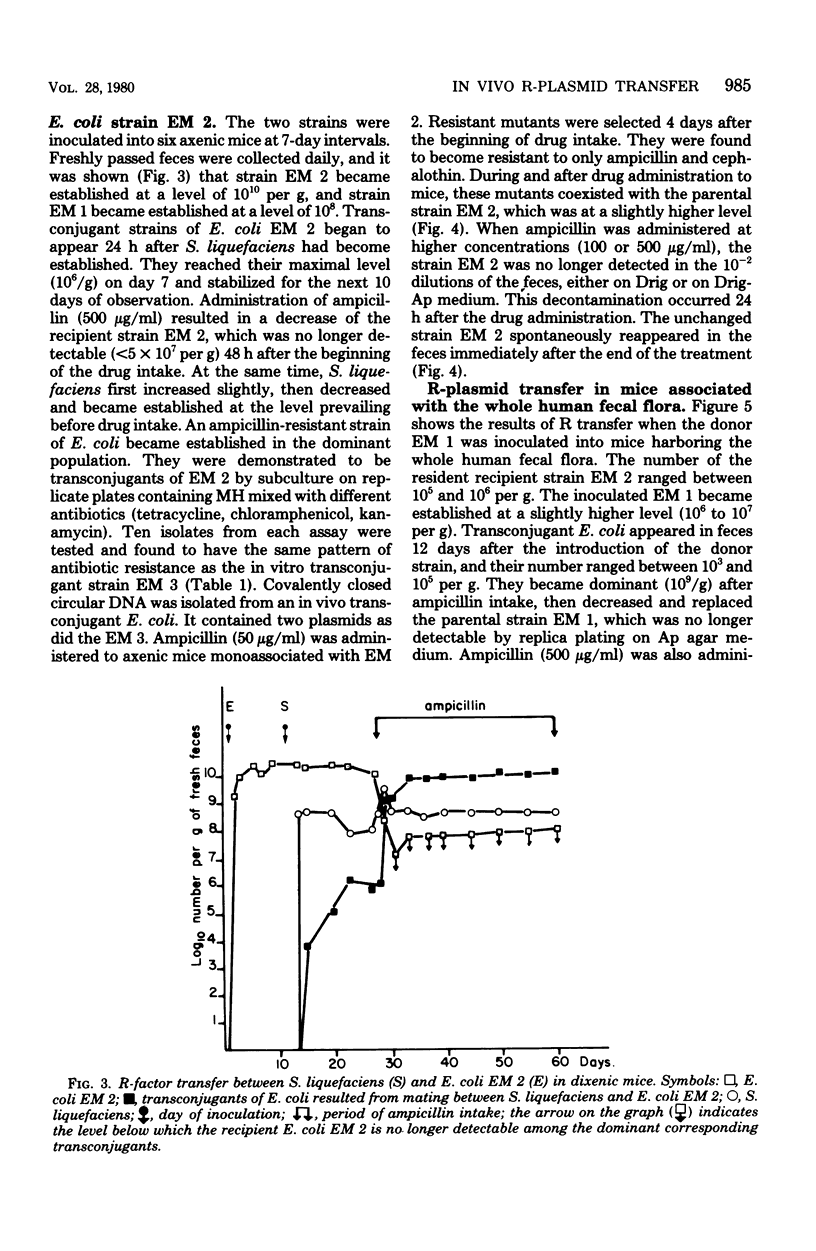
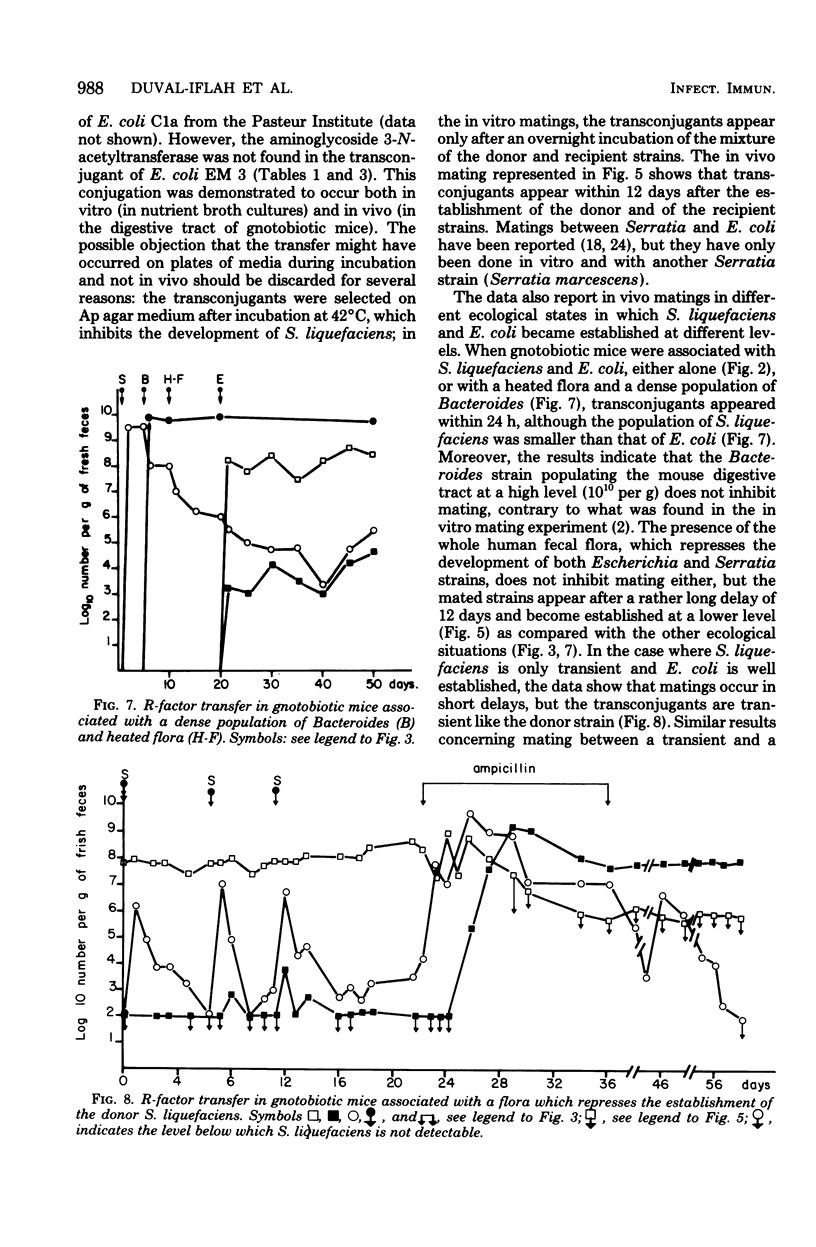
It was shown that a strain of Serratia liquefaciens harbors a conjugative R-plasmid responsible for reistance to the following 14 antibiotics: ampicillin, carbenicillin, cephalothin, butirosin, neomycin, paramomycin, kanamycin, lividomycin, gentamicin, tobramycin, streptomycin, tetracycline, sulfonamide, and chloramphenicol, which belong to five families, the beta-lactamines, the aminoglycosides, the tetracyclines, the sulfonamides, and the phenicols. Resistance to th 14 antibiotics was cotransferred by in vitro conjugation between S. liquefaciens and strains of Escherichia coli. Mating between S. liquefaciens and E. coli also occurred in vivo, in the digestive tract of axenic mice and gnotobiotic mice associated with the whole human fecal flora. It was also shown that mating between these two strains occurred even when the donor S. liquefaciens strain was only transient in the digestive tract of the gnotobiotic host animals. A dense population of Bacteroides (10(10) viable cells per g of fresh feces) did not hinder this mating. All the matings occurred in the absence of an antibiotic selection pressure, and the resulting transferred strain of E. coli did not have the same colonizing capacity as the recipient parental strain. However, during antibiotic administration to mice, and even after the end of the drug intake, the transconjugant became established in the dominant population and replaced the parental recipient strain.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. D. Factors that may prevent transfer of anti-biotic resistance between gram-negative bacteria in the gut. J Med Microbiol. 1975 Feb;8(1):83–88. doi: 10.1099/00222615-8-1-83. [DOI] [PubMed] [Google Scholar]
- Anderson J. D. The effect of R-factor carriage on the survival of Escherichia coli in the human intestine. J Med Microbiol. 1974 Feb;7(1):85–90. doi: 10.1099/00222615-7-1-85. [DOI] [PubMed] [Google Scholar]
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
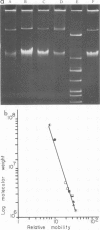
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Ducluzeau R., Rapine P., Courvalin C., Raibaud P. Transfert de la flore microbienne fécale de porcelets et de porcs adultes holoxéniques à des souris adultes et des porcelets axéniques : effet de l'animal hôte et du régime alimentaire sur le faciès microbien du tube digestif des divers animaux. Ann Microbiol (Paris) 1978 Nov-Dec;129 B(4):597–612. [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- KASUYA M. TRANSFER OF DRUG RESISTANCE BETWEEN ENTERIC BACTERIA INDUCED IN THE MOUSE INTESTINE. J Bacteriol. 1964 Aug;88:322–328. doi: 10.1128/jb.88.2.322-328.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocheilou V., Grinsted J., Richmond M. H. R-plasmid transfer in vivo in the absence of antibiotic selection pressure. Antimicrob Agents Chemother. 1976 Oct;10(4):753–761. doi: 10.1128/aac.10.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocheilou V., Richmond M. H., Bennett P. M. Spread of a single plasmid clone to an untreated individual from a person receiving prolonged tetracycline therapy. Antimicrob Agents Chemother. 1977 Aug;12(2):219–225. doi: 10.1128/aac.12.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud P., Dickinson A. B., Sacquet E., Charlier H., Mocquot G. La microflore du tube digestif du rat. I. Techniques d'étude et milieux de culture proposés. Ann Inst Pasteur (Paris) 1966 Apr;110(4):568–590. [PubMed] [Google Scholar]
- Richmond A. S., Simberkoff M. S., Rahal J. J., Jr, Schaefler S. R factors in gentamicin-resistant organisms causing hospital infection. Lancet. 1975 Dec 13;2(7946):1176–1178. doi: 10.1016/s0140-6736(75)92660-4. [DOI] [PubMed] [Google Scholar]
- Salzman T. C., Klemm L. Transfer of antibiotic resistance (R factor) in the mouse intestine. Proc Soc Exp Biol Med. 1968 Jun;128(2):392–394. doi: 10.3181/00379727-128-33020. [DOI] [PubMed] [Google Scholar]
- Smith H. W. Transfer of antibiotic resistance from animal and human strains of Escherichia coli to resident E. coli in the alimentary tract of man. Lancet. 1969 Jun 14;1(7607):1174–1176. doi: 10.1016/s0140-6736(69)92164-3. [DOI] [PubMed] [Google Scholar]
- Walton J. In vivo transfer of infectious drug resistance. Nature. 1966 Jul 16;211(5046):312–313. doi: 10.1038/211312a0. [DOI] [PubMed] [Google Scholar]