Abstract
Type 2 immunity encompasses the mechanisms through which the immune system responds to helminths and an array of environmental substances such as allergens. In the developing world, billions of individuals are chronically infected with endemic parasitic helminths. In comparison, in the industrialized world, millions of individuals suffer from dysregulated type 2 immunity, referred to clinically as atopic diseases including asthma, allergic rhinitis and atopic dermatitis. Thus, type 2 immunity must be carefully regulated to mount protective host response yet avoid inappropriate activation and immunopathology. In this review, we describe the keys players and connections at play in type 2 responses and focus on the emerging mechanisms involved in the negative regulation of type 2 immunity.
The Unique Challenges of Type 2 Immunity
“Speak roughly to your little boy and beat him when he sneezes he only does it to annoy because he knows it teases.
I speak severely to my boy I beat him when he sneezes for he can thoroughly enjoy the pepper when he pleases”
Alice’s Adventures in Wonderland (1865), Lewis Carroll.
Type 2 immunity faces the challenge of dealing with parasitic helminths and molecules (such as allergens and toxins) capable of causing tissue damage. To confront these unique threats, the immune system has evolved a coordinated response of both immune and non-immune cells with the primary goal of expulsion and subsequent tissue repair (Box 1). However, when mounted against innocuous agents (allergens) or exaggerated, this response can harm the host. In addition, type 2 immunity has recently been connected to adipose tissue remodeling and metabolism (Box 2), an aspect we will not focus on in this review. Rather, we will concentrate on the features of type 2 immunity such as the innate immune sensors or the negative regulators that control the amplitude and duration of the response.
Box 1. Characteristics and Modeling of Parasitic Helminth Infections.
An important characteristic of helminths is that, for the most part, they are unable to complete their life cycle entirely in a single host. This stands in contrast to other microbes that are able to proliferate within the host and overwhelm the immune system through sheer numbers. This fundamental difference in the nature of the immune threat allows the immune system to develop a strategy of tolerance. This tolerance is different from immune tolerance, or unresponsiveness to self-antigens, and belongs of a set of host defense strategies that include resistance and tolerance. Briefly, resistance comprises mechanisms that protect the host by reducing the pathogen burden. In contrast, tolerance mechanisms do not affect the pathogen burden but instead promote survival by limiting the negative impact of an infection on host fitness [112–114]. Additionally, in natural conditions, individuals are exposed to low doses of parasites but because helminths are endemic, exposure is often repeated. This places an increased importance on a robust memory response to prevent reinfection and limit parasite burden. Much of our current knowledge of type 2 immune responses stems from the use of model organisms such as Nippostrongylus brasiliensis, Heligmosomoides polygyrus or Schistosoma mansoni. Most experimental protocols include a single exposure with a relatively large dose of parasites, which differs from the low dose, repeated exposure characteristic of natural infections. Future studies might benefit from considering the environmental conditions behind the evolution of type 2 immune response mechanisms.
Box 2. Metabolism and Type 2 Immunity.
Adipose tissue is an important metabolic organ. It can be functionally divided into white adipose tissue (WAT) and brown adipose tissue (BAT). BAT is highly catabolic and plays an important role in thermogenesis while WAT is associated with anabolic metabolism and serves as energy storage [115]. Recently, it has become apparent that a number of type 2 immune cells and pathways are also involved in the regulation of adipose tissue metabolism (reviewed in [115–117]). Briefly, macrophages are an abundant immune cell population in adipose tissue. During homeostasis, they are under the control of IL-4 and IL-13, produced by eosinophils and ILC2s respectively [118–120]. Exposure to these cytokines initiates a program in macrophages that supports differentiation of adipocyte precursors, increases insulin sensitivity, and triggers the beiging of WAT, a process through which it acquires BAT-like characteristics such as thermogenesis [115–117]. Tregs are also present in the fat and further support this program in macrophages [115, 121]. This process is an active area of research and several questions remain. In particular, although IL-33 has been implicated, the source of the activating signals for this pathway is unclear [117]. The evolutionary significance behind the link, and indeed the specifics of the link itself, between type 2 immunity and control of metabolism remains unclear.
Induction of Type 2 Immune Responses
Detection, a New Role for Epithelia
As the site of first encounter with parasites or allergens, the epithelium has recently been highlighted as a central player in the initiation of type 2 immunity (Figure 1). Exposure to allergens and helminths, induces the production of cytokines such as thymic stromal lymphopoietin (TSLP) by epithelial cells [1]. TSLP signals to dendritic cells (DCs) to express type 2 helper T cells (Th2)-recruiting chemokines, and drives DCs to skew naïve T cells toward the Th2 phenotype [2]. In individuals with atopic dermatitis, TSLP is produced at increased levels in the affected epidermis compared to unaffected sites underscoring its role in initiation of type 2 immunity [3].
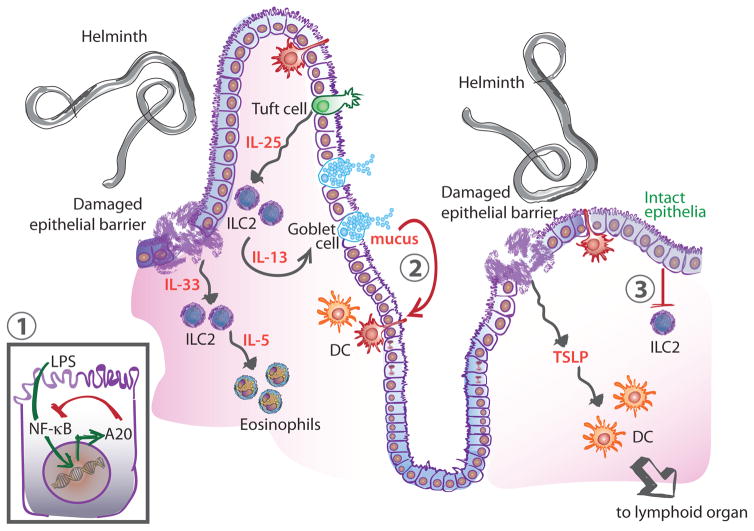
Figure 1. Regulation of Type 2 Immunity at Barrier Tissues.
Immune stimulus (e.g. helminth) leads to tissue damage and secretion of the cytokines IL-33 and TSLP. TSLP activates DCs to adopt a Th2-promoting phenotype and migrate to lymphoid organs to activate T cells. IL-33 signals on ILC2 leading to the recruitment of eosinophils through the secretion of IL-5. ILC2s also sense the increased production of IL-25 by tuft cells and secrete IL-13 which feeds back on goblet cells leading to increased mucus production. Negative regulatory mechanisms at the barrier include inhibition of NF-κB signaling in epithelial cells through the action of A20 (1). During homeostasis, mucus limits inflammation by inducing a regulatory pathway in DCs in the context of antigen uptake (2). Similarly, an intact epithelium provides immunoregulatory signals that limit ILC2 activation (3). Abbreviations: DC, dendritic cell; IL, interleukin; ILC2, type 2 innate lymphoid cell; TSLP, thymic stromal lymphopoietin.
Specialized epithelial cells, such as goblet and tuft cells, are also important in initiating type 2 immune responses. In the lung, goblet cells have been implicated in the initiation of type 2 inflammation under the control of the transcription factor SPDEF [4]. Tuft cells, rare intestinal epithelial cells, have been recently shown to produce basal levels of interleukin-25 (IL-25), a key cytokine in the maintenance of type 2 innate lymphoid cells (ILC2s) (Figure 1) [5–7]. In response to infection with the nematode Nippostrongylus brasiliensis, tuft cells undergo significant hyperplasia and increase production of IL-25, ultimately triggering expansion of effector ILC2s [5–7]. While the mechanism of recognition is still unclear, tuft cells express many receptors involved in the chemical sensing of taste, that have been suggested as potential sensors for parasites.
Whether originating from the skin, lungs or gut, type 2 immune responses rely on cytokines to coordinate the initial steps of the response. In addition to IL-25 and TSLP, another canonical mediator is IL-33. Exogenous IL-33 administration induces the characteristics signs of type 2 immune responses, such as IL-5 and IL-13 production, which in turn induce eosinophilia and mucus secretion, respectively (Figure 1) [8]. IL-33 is an important example of the role of damage detection in type 2 immunity, as it is released by injured or necrotic cells, while being degraded by caspases during apoptosis, earning it the title of “alarmin” [9].
Early Responders, Rapid Amplification
Local immune cells, such as ILC2s, propagate type 2 immune responses after initiation. Recently discovered and the subject of much study, they have been extensively reviewed elsewhere [10–13]. Enriched at barrier tissues, these cells lack markers associated with B cells, T cells, granulocytes, and myeloid cells. They do, however, rely on the same canonical transcription factors that define helper T cell subsets. For instance, ILC2s and Th2 cells both express the transcription factor GATA3 [10–13]. In response to IL-25, IL-33, and TSLP, ILC2s produce several effector type 2 cytokines, such as IL-5, IL-9, and IL-13 (Figure 1) [10–13]. Thus they allow a rapid, T cell-independent response to pathogens. ILC2s are also involved in the effector phase of the response and may play a role in T cell activation [14].
Other important early responders include granulocytes, such as mast cells in the tissue and basophils recruited from the circulation. In response to signaling from pattern recognition receptors or cytokines, including TSLP or IL-33, they undergo degranulation releasing preformed factors including proteases, histamine, IL-4, and tumor necrosis factor, amplifying type 2 inflammation locally through edema and further recruitment of leukocytes [15, 16]. Of note, these cells also play an important role in effector and memory responses through their ability to bind the antibody isotypes generated in type 2 responses.
Initiation of Adaptive Immunity, Fog of War
The cornerstone of adaptive immunity is the activation and differentiation of helper T cells into subsets specific for the pathogen encountered. Parasites and allergens lead to the development of GATA3+ Th2 cells that produce IL-4, IL-5, IL-9, and IL-13 [17] and, in combination with ILC2s, coordinate the effector response (described later). Th9 cells form another helper subset associated with allergies and anti-helminth immunity (reviewed in [18, 19]). In addition, specialized follicular helper T cells (Tfh) trigger the maturation of B cells to plasma cells [20] and, through the sequential secretion of IL-21 and IL-4 [21], drive class switch recombination to IgE and IgG1, the major subsets of immunoglobulins in type 2 immunity.
As with all T cells, Th2 activation is dependent on three signals provided by professional antigen presenting cells (APCs) such as DCs (Figure 2). First, DCs present T cells with their cognate peptide antigens in the context of the major histocompatibility complex (MHC). Studies of these early signaling events have shown that short-term or low avidity interactions between T cells and APCs favor Th2 differentiation [22]. The second signal, referred to as costimulation, confirms and enhances T cell activation. Certain costimulatory molecules have also been shown to skew T cell differentiation. Several groups have shown that, in response to TSLP, DCs upregulate the costimulatory molecule OX40 ligand (OX40L) [2, 3]. T cells sense OX40L through the OX40 receptor and produce IL-4, IL-5, and IL-13 in response [2, 23].
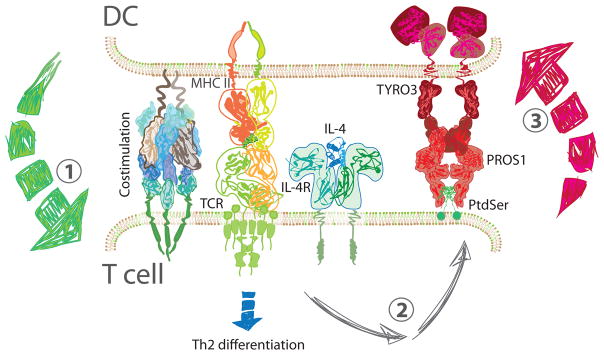
Figure 2. Regulation at the Immune Synapse.
CD4+ T cells are activated and begin to differentiate in response to three signals (1). DCs present peptide antigen on the MHC class II and provide costimulation as well, to ensure productive signaling. Cytokines direct differentiation to specific CD4+ T helper subsets; here IL-4 leads to Th2 differentiation. In addition, the combination of TCR and IL-4 signaling in T cells leads to the upregulation of PROS1 on their surface (2). In turn, PROS1 interacts with TYRO3 on the DC and engages a negative feedback mechanism that limits DC activation (3). Abbreviations: DC, dendritic cell; IL-4, interleukin 4; IL-4R, interleukin 4 receptor; MCH II, class II major histocompatibility complex; PROS1, Protein S; PtdSer, phosphatidylserine; TCR, T cell receptor; Th2, type 2 helper T cell.
Signal 3, provided by the cytokine milieu, is perhaps the single most powerful determinant of immune skewing. IL-4 skews naïve T cells toward Th2, but despite the importance of this signal, its origin is an area of much debate because DCs have not been shown to produce IL-4 (Figure 2). Recently, much attention has been devoted to the identification of the Th2-inducing subset of DCs, leaving open the possibility of an IL-4-producing DC. The alternative hypothesis, in which IL-4 originates from a non-DC source, remains prevalent and is divided into two schools of thought. In one scenario, IL-4 is produced by T cells during activation and signals in an autocrine/paracrine fashion [24, 25]. In the other, an accessory cell, in conjunction with DCs, provides the necessary cues for Th2 differentiation. Candidates for these accessory cells include basophils and eosinophils [16, 26, 27]. However, the identity of this accessory cell is still highly debated and the induction of adaptive immunity in type 2 responses remains an area of intense study.
DCs are a heterogeneous population with subsets that preferentially induce distinct Th responses. It is clear that CD11c+ DCs are necessary for Th2 induction [28–30], yet no specific subset has been identified. Potential candidates are reviewed elsewhere [31, 32]. Several studies have demonstrated that DCs expressing the transcription factor interferon regulatory factor 4 (IRF4) can induce Th2 cells [33, 34], however, IRF4+ DCs can also induce Th17 cells [35]. More recently, Kruppel-like factor 4 (KLF4) has been suggested as another transcription factor to further specify the Th2-inducing population of IRF4+ DCs [36]. Much work has also been done to identify selective cell surface markers on Th2-inducing DCs. Programmed death ligand 2 (PD-L2), a co-inhibitory molecule, and macrophage galactose-type C-type lectin 2 (Mgl2 or CD301b) identify subsets of DCs capable of stimulating a memory Th2 response or dermal type 2 immunity respectively [33, 37]. Ultimately, it is likely that the type of challenge as well as its location dictate the DC subset necessary for Th2 cell activation.
Effector Mechanisms, Expulsion and Repair
Activated Th2 cells migrate to the affected tissue and work in concert with ILC2s to orchestrate the effector phase of the response through the secretion of IL-4, IL-5, IL-9, and IL-13 (Figure 3). IL-13 leads to goblet cell hyperplasia and increased mucus production, which helps with parasite expulsion [38]. Goblet cells also secrete molecules such as resistin-like molecule (RELM)-β that limit parasitic infection [39, 40]. Increased mucus production is often coupled with increased smooth muscle contractions driven by type 2 cytokines, a combination referred to as “weep and sweep” that enhances worm expulsion [41]. In addition to their local effect, these cytokines also recruit several effector populations.
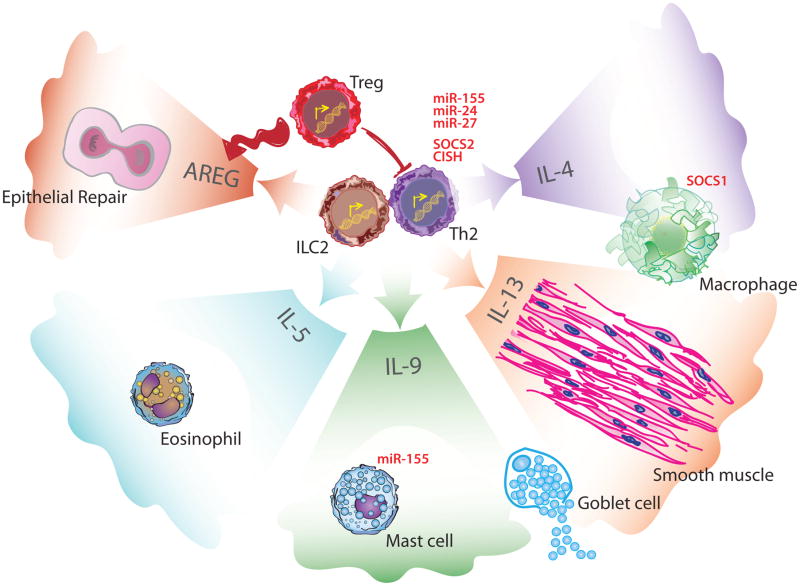
Figure 3. Regulation of Type 2 Effector Mechanisms.
During the effector phase of type 2 immunity, ILC2s and Th2 cells coordinate the response through the production of effector cytokines. In Th2 cells, the SOCS proteins CISH and SOCS2 have been shown to limit production of these effector cytokines. microRNAs such as miR-24, miR-27, and miR-155 also regulate Th2 cytokine production. IL-4 is primarily produced by Th2 and, in combination with IL-13, polarizes macrophages. This polarization is negatively regulated by SOCS1. ILC2s produce AREG to trigger epithelial growth and repair. Th2 and ILC2s are both capable of producing IL-5, IL-9 and IL-13. IL-5 contributes to eosinophils recruitment to the site of injury. Mast cells are recruited by IL-9 and miR-155 limits their degranulation. IL-9 and IL-13 act on goblets cells and lead to proliferation and increased mucus secretion. Finally, IL-13 signaling on smooth muscle cells leads to increased contractility. At the tissue level, these functions act together to promote expulsion of the inciting agent. Tregs are able to suppress Th2 cell activity and also contribute to tissue repair through AREG production. Abbreviations: AREG, amphiregulin; CISH, cytokine-inducible SH2-containing protein; IL, interleukin; ILC2, type 2 innate lymphoid cell; miR, microRNA; SOCS, suppressor of cytokine signaling; Th2, type 2 helper T cell; Treg, regulatory T cell.
ILC2 and Th9 cells are some of the most potent producers of IL-9 [42]. Like most cytokines, IL-9 is pleiotropic with effects in asthma, anti-helminth immunity as well as cancer and colitis [18]. In type 2 immunity, IL-9 contributes to mast cell recruitment, enhances ILC2 function, and promotes mucus secretion (Figure 3) [18, 19, 42]. Another relevant cytokine is IL-5 which, in combination with eotaxins, recruits eosinophils and induces eosinophilia, a common finding in parasitic infections and allergic conditions (Figure 3) [26]. Eosinophils were shown to kill helminth larvae in vitro [43, 44], however recent studies in eosinophil-deficient mice paint a more complex role for eosinophils in primary infection (reviewed in [26, 45]). In the case of secondary infections, data supports a protective function of eosinophils [45–47]. This memory response is particularly relevant as exposure to parasites in endemic areas is often recurrent and preventing reinfection limits host parasitic burden.
Th2 cells also recruit basophils through IL-3 secretion in both parasitic infections and allergic conditions [48]. IgE produced by plasma cells binds the high affinity IgE-specific receptor FcεRI expressed on mast cells and basophils which, when crosslinked, triggers degranulation. As with eosinophils, several studies show a protective effect of mast cells and basophils in secondary infections with parasitic helminths [49–51]. Basophils are also important in the pathogenesis of chronic atopic dermatitis [50].
Exposure to IL-4 and IL-13 skews macrophages, another effector population in type 2 immunity, toward a tissue repair phenotype characterized by the expression of factors such as the enzyme arginase-1 (Arg-1), the chitinase-like protein Ym1, and Relm-α [52]. The biology of macrophages in type 2 immunity is complex yet some patterns can be discerned. In Schistosoma mansoni (S. mansoni) infection, parasitic eggs are deposited in the liver and trigger the formation of granulomas. Macrophages play a key role in this granulomatous response and several groups have shown that they originate from circulating monocyte precursors [53, 54]. Similarly, IL-4/IL-13 polarized macrophages found in the large intestine of mice infected with the whipworm Trichuris muris (T. muris) are also derived from monocyte precursors [55]. In contrast, the IL-4/IL-13-polarized macrophages found in the thoracic cavity of mice infected by the filarial nematode Litomosoides sigmodontis (L. sigmodontis) are not derived from monocytes recruited to the site of damage, but rather proliferate and differentiate from tissue resident precursors [56]. This pattern is also seen in the peritoneum of Heligmosomoides polygyrus (H. polygyrus) infected mice [57]. Thus, it is currently thought that certain inflammatory conditions, such as those present in the colon of T. muris infected mice, lead to the recruitment of circulating monocytes that will be the predominant source of IL-4/IL-13-polarized macrophages. Conversely, inflammatory conditions found in the pleural and peritoneal cavity during L. sigmodontis and H. polygyrus infection, respectively, favor the expansion and polarization of tissue resident macrophages [58]. Just as their origin is complex, the contribution of IL-4/IL13-polarized macrophages to anti-helminth immunity remains unclear and is perhaps parasite-specific. In the context of H. polygyrus infection, macrophages in the intestinal mucosa are polarized by memory Th2 cells and provide for immunity to reinfection [59]. Using macrophage depletion experiments in N. brasiliensis infected mice, researchers have shown that macrophages do not affect the initiation of the immune response but rather play a role in parasite expulsion and subsequent tissue repair [60, 61]. Similarly, IL-4/IL-13-polarized macrophages are thought to be protective in S. mansoni infection by limiting liver fibrosis [62, 63]. These last examples highlight the importance of tissue repair in parasitic infections. Helminths cause significant tissue damage and a repair response is a crucial tolerance mechanism, although it is not unique to type 2 immunity [61]. Interestingly, IL-4/IL-13-polarized macrophages also play a role in the regulation of adipose tissue metabolism (Box 2).
Negative Regulation of Type 2 Immune Responses
Although our knowledge of type 2 immunity has increased exponentially over the past several years, its regulation remains poorly characterized. This lack of understanding is evident in the therapies used for atopic diseases. Initial treatment modalities, which remain regularly used, indiscriminately suppress inflammation through corticosteroids or prevent end-organ responses without addressing the underlying immune mechanisms (e.g. preventing bronchoconstriction with β-adrenergic agonists). Better understanding of the effector mechanisms of type 2 immunity has led to the development of more targeted therapies. Omalizumab, an antibody against IgE, is approved for patients with moderate to severe asthma or chronic idiopathic urticaria. Similarly, anti-IL-5 therapy using the monoclonal antibodies mepolizumab and reslizumab is effective in patients with severe asthma. More recently, dupilumab, an antibody against the IL-4 receptor α subunit has demonstrated strong efficacy in patients with atopic dermatitis [64]. Further characterization of the regulatory mechanisms of type 2 immune responses holds the key to the next generation of therapeutics as it would allow engagement of these pathways to normalize the magnitude of the dysregulated responses seen in atopic diseases. In contrast to the pronounced signs and symptoms of atopic conditions (such as asthma) or anaphylaxis, most helminth infections have subclinical or asymptomatic presentations. Explanations for this lack of symptoms include low parasite burdens (Box 1), as well as immune modulation triggered by the parasites [38, 41]. While helminth-derived products that regulate the immune response should not be ignored, we will focus on host-intrinsic, negative regulatory pathways in type 2 immunity for the remainder of the review. While expansive, this list is not meant to be exhaustive; instead we will strive to identify patterns and broad categories to highlight current gaps and future avenues of research.
Epithelium Regulation, Setting a New Threshold
The expanding role of epithelial cells in the initiation of type 2 immunity suggests that their regulation is a key control node. This notion has been supported by numerous studies that portray epithelium as limiting inflammation during homeostasis [65, 66]. This function of epithelium is logical when placed in context: in the gastrointestinal and respiratory tracts, single-layer epithelium must maintain barrier integrity while allowing efficient transfer of necessary nutrients and gases. Inflammation, and the subsequent tissue disruption, can severely impair organ function and thus must be curtailed. The mechanisms through which epithelial cells limit type 2 immune responses can be broadly divided into cis- and trans-acting mechanisms, as proposed by Weitnauer et al. [65].
Cis-acting mechanisms negatively regulate type 2 immunity by modulating intracellular signaling pathways in epithelial cells. This phenomenon is best understood in lung epithelium with NF-κB signaling although the activating stimuli are not well characterized. Specific inhibition of NF-κB activation in lung epithelial cells dampens allergic inflammation, highlighting the contribution of this pathway to pathology [67]. A recent study of asthma predisposition by Schuijs et al. offers a more precise mechanism: lung epithelial cells exposed to endotoxin found in farm dust upregulate the ubiquitin-modifying enzyme A20 downstream of NF-κB signaling [68]. In turn, A20 functions as a negative feedback mechanism, limiting NF-κB activation (Figure 1). In mice, the ablation of A20 in epithelial cells worsens inflammation in the house dust mite model of allergic lung disease. Interestingly, the authors also report reduced levels of A20 in bronchial epithelial cells of asthmatic patients. This study is particularly interesting because it provides an example of modulation of allergic disease with exogenous agents, which hold potential for therapeutic intervention. Other modulators of NF-κB signaling such as IL-1 receptor associated kinase-M (IRAK-M) have also been associated with asthma, but much work remains to be done to characterize their specific role [69]. In addition, whether similar mechanisms of regulation are at play in other barrier tissues remains to be examined.
Epithelial cells inhibit immune activation through trans-acting mechanisms as well. Signals originating from epithelial cells and indicating an intact, healthy barrier limit immune cell activation. For instance, MUC2, a key component of mucus in the intestinal tract binds antigens through sugar moieties. When taken up by dendritic cells, MUC2-coated antigens induce specific downregulation of inflammatory signals while leaving production of regulatory cytokines unchanged (Figure 1) [70]. Thus, antigens encountered in the context of an intact barrier do not trigger immune activation. While this pathway is not unique to type 2 immunity, the fundamental role played by epithelia in initiating type 2 immune responses suggests it is a likely mechanism of regulation. Additionally, epithelial cells provide a physical barrier through their tight and adherens junctions. It was recently shown that E-cadherin, a cell adhesion junction molecule, binds killer-cell lectin-like receptor G1 (KLRG1) on ILC2s and inhibits the production of IL-5 and IL-13 (Figure 1) [71]. Presumably, when disrupted, the epithelial barrier becomes unable to provide baseline regulatory signals, lowering the threshold for innate cell activation, but this remains to be established. Once tissue is repaired, feedback between the epithelium and immune system is likely to be an important contributor in the termination of the active immune response.
Regulation of Cytokine Signaling in Innate Type 2 Immunity, Peaking Behind the Curtain
Cytokines are critical orchestrators of the innate response. They classically exert their effect through Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling, which is negatively regulated by suppressor of cytokine signaling (SOCS) proteins. Although this mechanism has been well defined in other immune responses, the contribution of SOCS-mediated regulation in type 2 immunity is not well characterized [72]. In macrophages, SOCS1 has been implicated in the regulation of the IL-4/IL-13-induced phenotype, yet it is also involved in regulating IFNγ signaling, making it difficult to ascertain its precise function (Figure 3) [72]. In DCs, STAT5 is involved in the TSLP-mediated induction of Th2 cells, but the effects of SOCS proteins on this pathway have not been investigated [73]. Even less is known about the role SOCS proteins in granulocytes or ILC2s. This area of study is likely to yield interesting results, as ILCs are particularly dependent on cytokine signaling for their activation and function. Another interesting mechanism of regulation of cytokine function is the IL-13 receptor α2 subunit (IL-13Rα2). IL-13 signals through the type 2 IL-4 receptor, which is composed of two subunits, IL-4 receptor α and IL-13Rα1 [74]. As the name indicates, the type 2 IL-4 receptor also responds to IL-4. In contrast, IL-13Rα2 binds IL-13 exclusively and with higher affinity than the type 2 IL-4 receptor and its cytoplasmic domain lacks a signaling motif [75]. Several papers have demonstrated a role for IL-13Rα2 as a decoy receptor able of limiting IL-13-mediated responses both in allergic and parasitic diseases [76–78]. IL-13Rα2 expression is dependent of IL-13 signaling suggesting it is an endogenous, negative feedback mechanism to control IL-13-induced pathology [74].
microRNAs, (Relative) Newcomers in Type 2 Immunity Regulation
microRNAs (miRNAs) are small (~22 nucleotides) endogenous RNAs that influence gene expression either through directed mRNA degradation or translational repression. A single miRNA can target many genes and single genes can be regulated by multiple miRNAs. As such, miRNAs are primed to affect entire gene networks and have been described as master regulators [79]. Their regulatory contribution to type 2 immunity has been reviewed elsewhere [80], so we will focus on one example to highlight the complexity of miRNA-mediated regulation. miR-155 is upregulated in ILC2s in response to IL-33 exposure and is needed for their subsequent expansion [81]. Yet, in mast cells, miR-155 expression inhibits degranulation and cytokine production (Figure 3) [82]. Thus, while there is clear evidence of involvement of miR-155 in type 2 immunity, its effects vary greatly in different cells or conditions.
Regulation of Adaptive Type 2 Immune Responses
Th2 cells are key coordinators of the effector response in type 2 immunity. Yet, the mechanisms through which Th2 cells are negatively regulated remain poorly understood. Several SOCS proteins have been shown to inhibit Th2 responses (Figure 3). Cytokine inducible SH-2 containing protein (CISH), a member of the SOCS family, was recently shown to be upregulated in T cells in response to IL-4 [83]. Through its inhibition of STAT3, STAT5, and STAT6, CISH prevents allergic airway inflammation [83]. SOCS2 has also been implicated in the regulation of Th2 cells as its deficiency in T cells leads to enhanced type 2 cytokine secretion during helminth responses as well as worsened inflammation in allergic lung models [84]. miRNAs also play a role in regulating Th2 function (Figure 3). In vitro, miR-155 prevents T cell secretion of IL-4 through downregulation of c-Maf [85, 86]. Other miRNAs, such as miR-24 and miR-27, can also affect IL-4 production [87]. Despite the identification of specific agents, the mechanisms of miRNA-mediated regulation of helper T cell differentiation remains poorly characterized [88]. While individual mechanisms of Th2 negative regulation have been identified, their specific roles and coordination during a physiological type 2 immune response remain unclear. In addition, little is known about the negative regulation of B cells in type 2 immunity.
Regulation at the Interface of Innate and Adaptive Immunity
Innate and adaptive immunity collaborate tremendously during type 2 immune responses. The interaction of these two arms of the immune system must be tightly controlled to minimize the risk of exuberant inflammation. Two important examples of negative regulation that encompasses these two arms include regulatory T cells (Tregs) (discussed below) and inhibitory pathways at the DC-T cell synapse. Because of its role in the activation of the adaptive immune response, the interaction of DC and T cells requires careful modulation. As discussed above, the OX40L-OX40 pathway leads to Th2 skewing. Despite the critical role of OX40L and OX40, little is known about mechanisms that control their function. Other costimulatory and co-inhibitory molecules have emerged as targets in the context of cancer immunotherapy. Studies focused on the immune related adverse events associated with their pharmacological manipulation might shed light on their contribution to the negative regulation of type 2 immunity.
T cells and DCs also interact through mechanisms other than costimulation. One such example is the interaction between Protein S (PROS1) and the TYRO3, AXL, and MERTK (TAM) receptors on DCs. During T cell activation, PROS1 is expressed by T cells and signals through the TAM receptors on DCs leading to negative regulation of the immune response [89]. Interestingly, while PROS1 is transiently expressed in Th1 and Th17 cells following T cell activation, it is maintained in Th2 cells, as a result of sustained IL-4 signaling [90]. The maintenance of PROS1 on Th2 cells further underlines the importance of negative regulation in type 2 immune responses. PROS1 interacts with TYRO3 on DCs to specifically inhibit type 2 inflammation, both in allergic and anti-helminth responses in mice (Figure 2) [90]. In humans, genome wide association studies have revealed a link between TYRO3 and asthma in African American and Latino patients, but the mechanisms remain unclear [90].
Tregs, New Tricks for an Old Dog
Since their discovery, Tregs have been associated with the negative regulation of immune responses. Their importance in regulating type 2 inflammation is evident from the first reports of patients with mutation in the canonical Treg transcription factor, FOXP3 [91]. In addition to several autoimmune abnormalities, patients presented with elevated IgE, eosinophilia, dermatitis, and food allergies, all of which are type 2 immunity-dependent. The details of Treg control of type 2 immunity have been a major question in the field. Tregs can be divided into effector subtypes that match helper T cell subsets. The expression of similar transcription factors between effector T cells and Tregs is thought to lead to expression of similar chemokine receptors allowing co-localization and more effective regulation [92]. In that regard, IRF4-expressing Tregs have been shown to specifically control type 2 immune responses [93]. Tregs are either derived from the thymus during T cell selection or induced in the periphery when exposed to transforming growth factor-β in addition to T cell receptor (TCR) signaling. These induced Tregs (iTregs) are dependent on the FOXP3 enhancer CNS1 [94]. Using CNS1-deficient mice, Josefowicz et al. show that iTregs are essential to the control of Th2 response at mucosal sites [94]. The recent work of Bacher et al. further expands on this notion that Tregs are actively involved in the regulation of Th2 responses. Using novel techniques to enrich rare antigen-activated T cells, they were able to identify a robust pool of activated Tregs that prevent Th2 responses to common inhaled allergens [95]. Interestingly, they report that these Tregs are fully functional in allergic patients suggesting that the aberrant activation of Th2 cells is responsible for the allergic response in these individuals. These reports once again draw attention to the mucosa as a site important not only for the induction but also for the negative regulation of type 2 immunity.
Two important mechanisms proposed for Treg function involve the expression of the co-inhibitory molecule cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and production of the immunoregulatory cytokine IL-10. Treg-specific ablation of these molecules has revealed interesting contributions of these mechanisms to immune regulation. While the Treg-specific deletion of CTLA-4 leads to fatal lymphoproliferative disease, deletion of IL-10 in Tregs instead leads to increased inflammation at barrier tissues. This presents not only as colitis but also as allergic lung inflammation and contact hypersensitivity, two conditions associated with dysregulated type 2 immunity [96, 97]. In addition to CTLA-4, Tregs express other co-inhibitory molecules, such as TIGIT. Recently, Joeller et al. showed that TIGIT allows Tregs to specifically regulate type 1 and type 3 immune responses raising the interesting possibility that there exists similar co-inhibitory molecule(s) involved in specific control of type 2 immunity [98]. The role of Tregs in controlling type 2 immune responses, particularly at mucosal surfaces, has become more apparent, but much work remains.
Tissue Repair, a Special Connection to Helminth Infections?
As previously described, helminths cannot accomplish their entire life cycle within their host, making tolerance a viable immune strategy. This disease tolerance is mainly achieved through two mechanisms, tissue damage control and tissue repair [99]. Of these two, tissue repair has been most commonly associated with type 2 immunity [61, 100]. Yet, damage—whether caused by a pathogen or immunopathology—is a component of all immune responses and, as such, it is difficult to attribute the subsequent tissue repair specifically to a type of immune response. During the course of a type 2 immune response, IL-4 and IL-13 induce important tissue repair mechanisms, particularly through macrophage effector functions as previously described. Amphiregulin (AREG) is an example of an effector molecule in tissue repair responses. Tregs are involved in tissue repair through the production of Areg in a model of viral infection; however this has yet to be demonstrated in a type 2 immune response [101]. Similarly, ILC2-produced AREG is essential to the tissue repair process after influenza infection [102]. This last example is particularly striking since it involves effector cells from type 2 immunity in the repair of damage induced in the context of type 1 immune responses illustrating the unique connection between tissue repair and type 2 immunity.
Inhibition of damage-inducing inflammation enhances the efficacy of tissue repair and it has been proposed that tissue repair mechanisms actively suppress the ongoing immune response. Ablation of Arg-1 in macrophages in a model of S. mansoni infection leads to increased production of type 2 cytokines and fatal hepatic fibrosis [63]. Similarly, Relm-α-deficient mice challenged with S. mansoni eggs show increased fibrosis and granuloma burden in the lung, as well as exuberant production of type 2 cytokines by Th2 cells [103]. The interaction between tissue repair and inflammatory mechanisms in type 2 immunity is poorly studied, with perhaps the exception of S. mansoni infection. Yet, it is a highly relevant interaction since the natural course of helminth infections, and atopic responses as well, is often chronic and thus features overlap of repair and expulsion mechanisms.
Lessons and Limitations of Human Diseases
In addition to mutations in FOXP3, several human immunodeficiencies present with elevated IgE and eosinophilia and are classified as hyper IgE syndromes (HIES) [104]. These rare diseases provide an invaluable window into mechanisms of negative regulation of type 2 immunity in humans. One such syndrome, dedicator of cytokinesis 8 protein (DOCK8) deficiency, has revealed key players and also highlighted limitations to the studies of human diseases. DOCK8 deficiency, also classified as autosomal recessive HIES, presents with eczema, allergies (particularly to food allergens) and recurrent infections in addition to elevated IgE and eosinophilia [105]. DOCK8-deficient T cells have an intrinsic bias toward a Th2 phenotype as well as defects in Th17 differentiation through reduced STAT3 activity [106, 107]. The impaired Th17 differentiation is notable because it replicates a finding in the autosomal dominant form of HIES (AD-HIES, also known as Job’s syndrome) associated with mutations in STAT3 [108]. Interestingly, studies of the antigen specificities for IgE in HIES have revealed stark differences between the two syndromes. In DOCK8 deficiency, most antibodies react to food allergens while IgE antibodies from AD-HIES patients showed minimal reactivity against common oral and inhaled allergens [109]. To date, it is still unclear how a defect in Th17 immunity leads to HIES features. These patients also suffer from recurrent skin and pulmonary infections, a consequence of their immunodeficiency and perhaps these recurrent barrier disruptions trigger inappropriate type 2 immune activation. Similarly, recent studies by Janssen et al. have shown an association between DOCK8 deficiency and another syndrome associated with eczema, elevated serum IgE levels, and food allergies, Wiskott-Aldrich syndrome (WAS) [110]. Both DOCK8 and the WAS protein interact to support the cytoskeletal changes that occur in T cell activation thus potentially explaining the clinical overlap between the two syndromes [110]. Additionally, DOCK8-deficient patients suffer from defects in immune tolerance as shown by the increase in B cells reacting to self-antigens as well as defects in Treg number and function [111]. These examples highlight the difficulties in interpreting the clinical presentation of these diseases. As discussed previously, regulation involves multiple cell types and mechanisms, all of which can be affected as a consequence of the germline nature of these mutations. The pleiotropic effects of mutated molecules, as evidenced by the other symptoms of these syndromes adds an additional layer of complexity and makes interpretation difficult without further basic research in animal models.
Concluding Remarks
As our knowledge of type 2 immunity increases, so does our appreciation for the importance of its negative regulation. Yet, we are only now beginning to unravel its mechanisms (see Outstanding Questions). As the site of initial encounter, epithelial barriers are a central location for the initiation, effector and repair phases of type 2 immunity. Epithelial cells are emerging as central players in innate regulation of type 2 responses, but how they accomplish this function is still unclear. Similarly, while the role of cytokines in the initiation of the response is well established, more work is needed to uncover how they are specifically regulated in type 2 immunity. Studies of Treg biology have revealed selective mechanisms of regulation, particularly at mucosal surfaces but other regulatory pathways are yet to be discovered in adaptive type 2 immunity, particularly with respect to B cells. The interface between adaptive and innate immunity has emerged as a crucial node of negative regulation because of its potential for modulation of both arms of type 2 immune responses. These mechanisms of negative regulation primarily affect the resistance to the pathogen. Tolerance, and the associated tissue repair processes, is another important response to infection and is also triggered during type 2 immunity. The interactions between these two responses are just beginning to be understood. Elucidation of these mechanisms will provide a better understanding of the immune response to parasitic infections as well as provide potential treatments for individuals suffering from unchecked type 2 pathology.
Outstanding questions.
As the molecular and cellular sensors of helminths, allergens, and other stimuli of type 2 immunity are better defined, it will be interesting to explore if there exist dedicated negative regulators for these sensing pathways. Should this be the case, could this knowledge lead to targeted therapeutic interventions?
Co-infection with helminth and bacteria or viruses is common in the developing world. Does the engagement of negative regulatory pathways of type 2 immunity impact the progression of the co-infection?
Even though granulocytes such as eosinophils, mast cells, and basophils have long been associated with type 2 immunity, how they are negatively regulated remains unclear. The same is true of much more recent players such as ILC2s.
Many of the cells types described in the disease tolerance response (e.g. ILC2s) are also key players in type 2 immunity. Do the mechanisms of negative regulation at work in these cells also affect their role in the tolerance response or is the latter regulated through different, specific mechanisms?
What is the overall contribution of these negative regulatory pathways in the context of a more natural, low parasite burden? How do they affect the memory response, which is critical in endemic regions with high re-exposure rates?
Trends Box.
Type 2 responses against parasitic helminths involve innate and adaptive immune cells as well as non-hematopoietic cells such as epithelial and smooth muscle cells.
Epithelium is increasingly recognized as the site of initiation of type 2 immunity as well as an important site of negative regulation.
Negative feedback from adaptive to innate immunity, particularly at the level of the T cell-DC interface, has emerged as a powerful mechanism of regulation in type 2 immunity.
Our understanding of regulatory T cells has increased tremendously in the past several years and has revealed subsets of regulatory T cells that specifically suppress type 2 immunity.
Acknowledgments
We would like to thank Helen A. Beilinson for inputs, revisions, and helpful comments to this manuscript. This work was supported by grants from the National Institutes of Health (NIH-NIAID R01 AI089824 to C.V.R. and S.G., T32 GM007205 to D.D.K), American Asthma Foundation (C.V.R.) and Alliance for Lupus Research (C.V.R.). C.V.R. is an HHMI Faculty Scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takai T. TSLP expression: cellular sources, triggers, and regulatory mechanisms. Allergol Int. 2012;61(1):3–17. doi: 10.2332/allergolint.11-RAI-0395. [DOI] [PubMed] [Google Scholar]
- 2.Ito T, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202(9):1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soumelis V, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 4.Rajavelu P, et al. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest. 2015;125(5):2021–31. doi: 10.1172/JCI79422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerbe F, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529(7585):226–30. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howitt MR, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351(6279):1329–33. doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Moltke J, et al. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529(7585):221–5. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Luthi AU, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31(1):84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Kim BS, Artis D. Group 2 innate lymphoid cells in health and disease. Cold Spring Harb Perspect Biol. 2015;7(5) doi: 10.1101/cshperspect.a016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17(7):765–74. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 12.Roediger B, Weninger W. Group 2 innate lymphoid cells in the regulation of immune responses. Adv Immunol. 2015;125:111–54. doi: 10.1016/bs.ai.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. 2015;21(7):698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliphant CJ, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41(2):283–95. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivera A, et al. Innate cell communication kick-starts pathogen-specific immunity. Nat Immunol. 2016;17(4):356–63. doi: 10.1038/ni.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voehringer D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol. 2013;13(5):362–75. doi: 10.1038/nri3427. [DOI] [PubMed] [Google Scholar]
- 17.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89(4):587–96. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan MH, et al. The development and in vivo function of T helper 9 cells. Nat Rev Immunol. 2015;15(5):295–307. doi: 10.1038/nri3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt E, et al. Th9 cells, new players in adaptive immunity. Trends Immunol. 2014;35(2):61–8. doi: 10.1016/j.it.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41(4):529–42. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinstein JS, et al. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. 2016;17(10):1197–1205. doi: 10.1038/ni.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamane H, Paul WE. Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunol Rev. 2013;252(1):12–23. doi: 10.1111/imr.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy. 2009;39(6):798–806. doi: 10.1111/j.1365-2222.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voehringer D, et al. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203(6):1435–46. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, et al. IL-2 and Autocrine IL-4 Drive the In Vivo Development of Antigen-Specific Th2 T Cells Elicited by Nematode Parasites. J Immunol. 2005;174(4):2242–9. doi: 10.4049/jimmunol.174.4.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg HF, et al. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13(1):9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang H, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11(7):608–17. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammad H, et al. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207(10):2097–111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phythian-Adams AT, et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207(10):2089–96. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plantinga M, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38(2):322–35. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Bouchery T, et al. The Differentiation of CD4(+) T-Helper Cell Subsets in the Context of Helminth Parasite Infection. Front Immunol. 2014;5:487. doi: 10.3389/fimmu.2014.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh KP, Mills KH. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013;34(11):521–30. doi: 10.1016/j.it.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Gao Y, et al. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity. 2013;39(4):722–32. doi: 10.1016/j.immuni.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams JW, et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun. 2013;4:2990. doi: 10.1038/ncomms3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Persson EK, et al. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013;38(5):958–69. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Tussiwand R, et al. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity. 2015;42(5):916–28. doi: 10.1016/j.immuni.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumamoto Y, et al. CD301b(+) dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity. 2013;39(4):733–43. doi: 10.1016/j.immuni.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maizels RM, et al. Susceptibility and immunity to helminth parasites. Curr Opin Immunol. 2012;24(4):459–66. doi: 10.1016/j.coi.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Artis D, et al. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci USA. 2004;101(37):13596–600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herbert DBR, et al. Intestinal epithelial cell secretion of RELM-β protects against gastrointestinal worm infection. The Journal of Experimental Medicine. 2009;206(13):2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11(6):375–88. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 42.Licona-Limon P, et al. Th9 Cells Drive Host Immunity against Gastrointestinal Worm Infection. Immunity. 2013;39(4):744–57. doi: 10.1016/j.immuni.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buys J, et al. The killing of newborn larvae of Trichinella spiralis by eosinophil peroxidase in vitro. Eur J Immunol. 1981;11(10):843–5. doi: 10.1002/eji.1830111018. [DOI] [PubMed] [Google Scholar]
- 44.Capron M, et al. In vitro killing of S. mansoni schistosomula by eosinophils from infected rats: role of cytophilic antibodies. J Immunol. 1979;123(5):2220–30. [PubMed] [Google Scholar]
- 45.Huang L, Appleton JA. Eosinophils in Helminth Infection: Defenders and Dupes. Trends Parasitol. 2016 doi: 10.1016/j.pt.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang L, et al. Eosinophils mediate protective immunity against secondary nematode infection. J Immunol. 2015;194(1):283–90. doi: 10.4049/jimmunol.1402219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knott ML, et al. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J Parasitol. 2007;37(12):1367–78. doi: 10.1016/j.ijpara.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Siracusa MC, et al. New paradigms in basophil development, regulation and function. Immunol Cell Biol. 2010;88(3):275–84. doi: 10.1038/icb.2010.1. [DOI] [PubMed] [Google Scholar]
- 49.Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J Immunol. 2010;184(1):344–50. doi: 10.4049/jimmunol.0901841. [DOI] [PubMed] [Google Scholar]
- 50.Ohnmacht C, et al. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33(3):364–74. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Herbst T, et al. Antibodies and IL-3 support helminth-induced basophil expansion. Proc Natl Acad Sci USA. 2012;109(37):14954–9. doi: 10.1073/pnas.1117584109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol. 2013;31:317–43. doi: 10.1146/annurev-immunol-032712-095906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Girgis NM, et al. Ly6C(high) monocytes become alternatively activated macrophages in schistosome granulomas with help from CD4+ cells. PLoS Pathog. 2014;10(6):e1004080. doi: 10.1371/journal.ppat.1004080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nascimento M, et al. Ly6Chi monocyte recruitment is responsible for Th2 associated host-protective macrophage accumulation in liver inflammation due to schistosomiasis. PLoS Pathog. 2014;10(8):e1004282. doi: 10.1371/journal.ppat.1004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Little MC, et al. Dynamic changes in macrophage activation and proliferation during the development and resolution of intestinal inflammation. J Immunol. 2014;193(9):4684–95. doi: 10.4049/jimmunol.1400502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jenkins SJ, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332(6035):1284–8. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jenkins SJ, et al. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J Exp Med. 2013;210(11):2477–91. doi: 10.1084/jem.20121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lauvau G, et al. Monocyte-mediated defense against bacteria, fungi, and parasites. Semin Immunol. 2015;27(6):397–409. doi: 10.1016/j.smim.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anthony RM, et al. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12(8):955–60. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borthwick LA, et al. Macrophages are critical to the maintenance of IL-13-dependent lung inflammation and fibrosis. Mucosal Immunol. 2016;9(1):38–55. doi: 10.1038/mi.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen F, et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18(2):260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vannella KM, et al. Incomplete deletion of IL-4Ralpha by LysM(Cre) reveals distinct subsets of M2 macrophages controlling inflammation and fibrosis in chronic schistosomiasis. PLoS Pathog. 2014;10(9):e1004372. doi: 10.1371/journal.ppat.1004372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pesce JT, et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5(4):e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simpson EL, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med. 2016 doi: 10.1056/NEJMc1700366. [DOI] [PubMed] [Google Scholar]
- 65.Weitnauer M, et al. Control of local immunity by airway epithelial cells. Mucosal Immunol. 2016;9(2):287–98. doi: 10.1038/mi.2015.126. [DOI] [PubMed] [Google Scholar]
- 66.Hammad H, Lambrecht BN. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity. 2015;43(1):29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 67.Broide DH, et al. Allergen-induced peribronchial fibrosis and mucus production mediated by IkappaB kinase beta-dependent genes in airway epithelium. Proc Natl Acad Sci USA. 2005;102(49):17723–8. doi: 10.1073/pnas.0509235102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schuijs MJ, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349(6252):1106–10. doi: 10.1126/science.aac6623. [DOI] [PubMed] [Google Scholar]
- 69.Balaci L, et al. IRAK-M is involved in the pathogenesis of early-onset persistent asthma. Am J Hum Genet. 2007;80(6):1103–14. doi: 10.1086/518259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shan M, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342(6157):447–53. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salimi M, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210(13):2939–50. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCormick SM, Heller NM. Regulation of Macrophage, Dendritic Cell, and Microglial Phenotype and Function by the SOCS Proteins. Front Immunol. 2015;6:549. doi: 10.3389/fimmu.2015.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bell BD, et al. The transcription factor STAT5 is critical in dendritic cells for the development of TH2 but not TH1 responses. Nat Immunol. 2013;14(4):364–71. doi: 10.1038/ni.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–56. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 75.Donaldson DD, et al. The murine IL-13 receptor alpha 2: molecular cloning, characterization, and comparison with murine IL-13 receptor alpha 1. J Immunol. 1998;161(5):2317–24. [PubMed] [Google Scholar]
- 76.Chiaramonte MG, et al. Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response. J Exp Med. 2003;197(6):687–701. doi: 10.1084/jem.20020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilson MS, et al. IL-13Ralpha2 and IL-10 coordinately suppress airway inflammation, airway-hyperreactivity, and fibrosis in mice. J Clin Invest. 2007;117(10):2941–51. doi: 10.1172/JCI31546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wood N, et al. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor alpha 2. J Exp Med. 2003;197(6):703–9. doi: 10.1084/jem.20020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149(3):515–24. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pua HH, Ansel KM. MicroRNA regulation of allergic inflammation and asthma. Curr Opin Immunol. 2015;36:101–8. doi: 10.1016/j.coi.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johansson K, et al. MicroRNA-155 is a critical regulator of type 2 innate lymphoid cells and IL-33 signaling in experimental models of allergic airway inflammation. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 82.Biethahn K, et al. miRNA-155 controls mast cell activation by regulating the PI3Kgamma pathway and anaphylaxis in a mouse model. Allergy. 2014;69(6):752–62. doi: 10.1111/all.12407. [DOI] [PubMed] [Google Scholar]
- 83.Yang XO, et al. The signaling suppressor CIS controls proallergic T cell development and allergic airway inflammation. Nat Immunol. 2013;14(7):732–40. doi: 10.1038/ni.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knosp CA, et al. SOCS2 regulates T helper type 2 differentiation and the generation of type 2 allergic responses. J Exp Med. 2011;208(7):1523–31. doi: 10.1084/jem.20101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thai TH, et al. Regulation of the germinal center response by microRNA-155. Science. 2007;316(5824):604–8. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 86.Rodriguez A, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316(5824):608–11. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pua HH, et al. MicroRNAs 24 and 27 Suppress Allergic Inflammation and Target a Network of Regulators of T Helper 2 Cell-Associated Cytokine Production. Immunity. 2016;44(4):821–32. doi: 10.1016/j.immuni.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baumjohann D, Ansel KM. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat Rev Immunol. 2013;13(9):666–78. doi: 10.1038/nri3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carrera Silva EA, et al. T cell-derived protein S engages TAM receptor signaling in dendritic cells to control the magnitude of the immune response. Immunity. 2013;39(1):160–70. doi: 10.1016/j.immuni.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chan PY, et al. The TAM family receptor tyrosine kinase TYRO3 is a negative regulator of type 2 immunity. Science. 2016;352(6281):99–103. doi: 10.1126/science.aaf1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Verbsky JW, Chatila TA. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases. Curr Opin Pediatr. 2013;25(6):708–14. doi: 10.1097/MOP.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11(2):119–30. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458(7236):351–6. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Josefowicz SZ, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482(7385):395–9. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bacher P, et al. Regulatory T Cell Specificity Directs Tolerance versus Allergy against Aeroantigens in Humans. Cell. 2016;167(4):1067–1078. e16. doi: 10.1016/j.cell.2016.09.050. [DOI] [PubMed] [Google Scholar]
- 96.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 97.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–58. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 98.Joller N, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40(4):569–81. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soares MP, et al. Tissue damage control in disease tolerance. Trends Immunol. 2014;35(10):483–94. doi: 10.1016/j.it.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 100.Gause WC, et al. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013;13(8):607–14. doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arpaia N, et al. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162(5):1078–89. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Monticelli LA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12(11):1045–54. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nair MG, et al. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J Exp Med. 2009;206(4):937–52. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Williams KW, et al. Eosinophilia Associated with Disorders of Immune Deficiency or Immune Dysregulation. Immunol Allergy Clin North Am. 2015;35(3):523–44. doi: 10.1016/j.iac.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Engelhardt KR, et al. The extended clinical phenotype of 64 patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2015;136(2):402–12. doi: 10.1016/j.jaci.2014.12.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Keles S, et al. Dedicator of cytokinesis 8 regulates signal transducer and activator of transcription 3 activation and promotes TH17 cell differentiation. J Allergy Clin Immunol. 2016;138(5):1384–1394. e2. doi: 10.1016/j.jaci.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tangye SG, et al. DOCK8-Deficient CD4+ T Cells are Biased to a Th2 Effector Fate at the Expense of Th1 and Th17 Cells. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Holland SM, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357(16):1608–19. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 109.Boos AC, et al. Atopic dermatitis, STAT3- and DOCK8-hyper-IgE syndromes differ in IgE-based sensitization pattern. Allergy. 2014;69(7):943–53. doi: 10.1111/all.12416. [DOI] [PubMed] [Google Scholar]
- 110.Janssen E, et al. A DOCK8-WIP-WASp complex links T cell receptors to the actin cytoskeleton. J Clin Invest. 2016;126(10):3837–3851. doi: 10.1172/JCI85774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Janssen E, et al. Dedicator of cytokinesis 8-deficient patients have a breakdown in peripheral B-cell tolerance and defective regulatory T cells. J Allergy Clin Immunol. 2014;134(6):1365–74. doi: 10.1016/j.jaci.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ayres JS, Schneider DS. Tolerance of infections. Annu Rev Immunol. 2012;30:271–94. doi: 10.1146/annurev-immunol-020711-075030. [DOI] [PubMed] [Google Scholar]
- 113.Medzhitov R, et al. Disease tolerance as a defense strategy. Science. 2012;335(6071):936–41. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8(11):889–95. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Odegaard JI, Chawla A. Type 2 responses at the interface between immunity and fat metabolism. Curr Opin Immunol. 2015;36:67–72. doi: 10.1016/j.coi.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12(1):15–28. doi: 10.1038/nrendo.2015.189. [DOI] [PubMed] [Google Scholar]
- 117.Cautivo KM, Molofsky AB. Regulation of metabolic health and adipose tissue function by group 2 innate lymphoid cells. Eur J Immunol. 2016;46(6):1315–25. doi: 10.1002/eji.201545562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nussbaum JC, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502(7470):245–8. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qiu Y, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157(6):1292–308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu D, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332(6026):243–7. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feuerer M, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]