Abstract
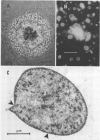
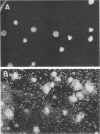
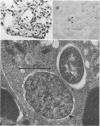
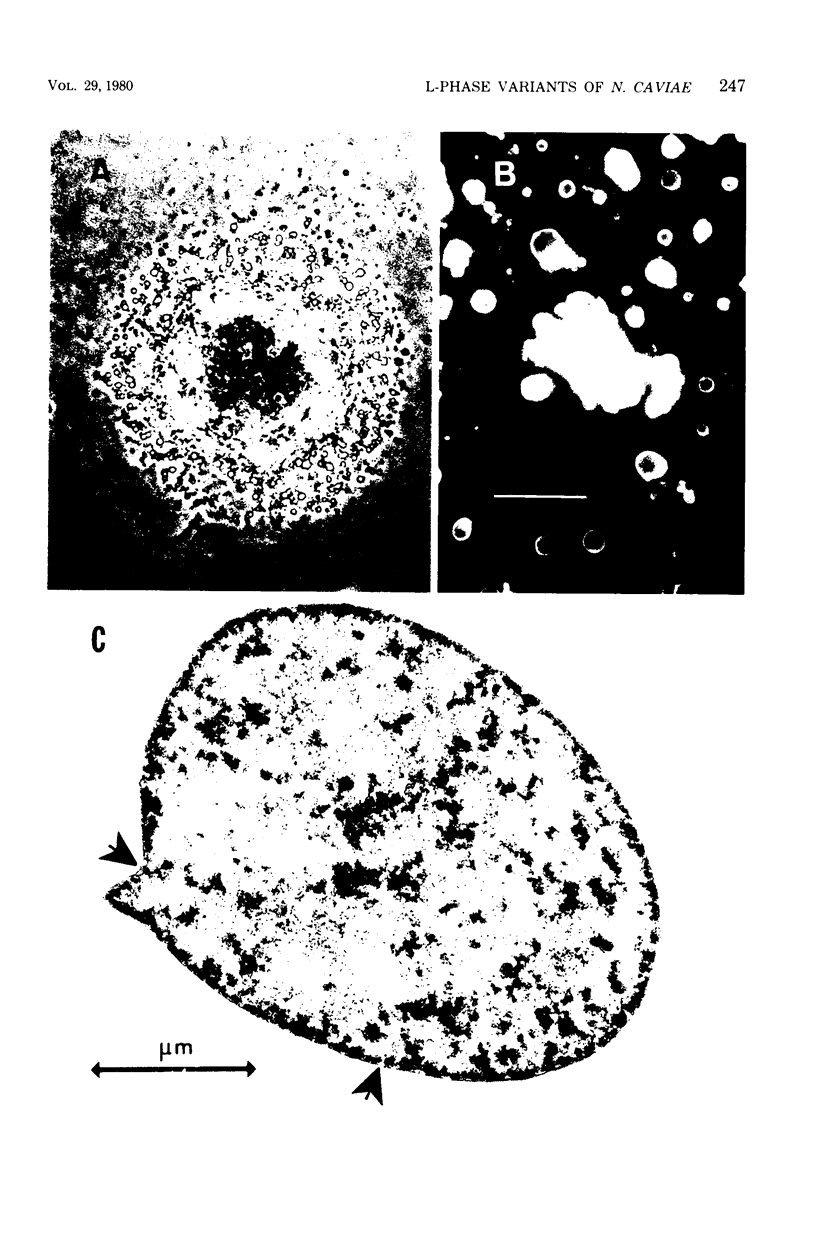
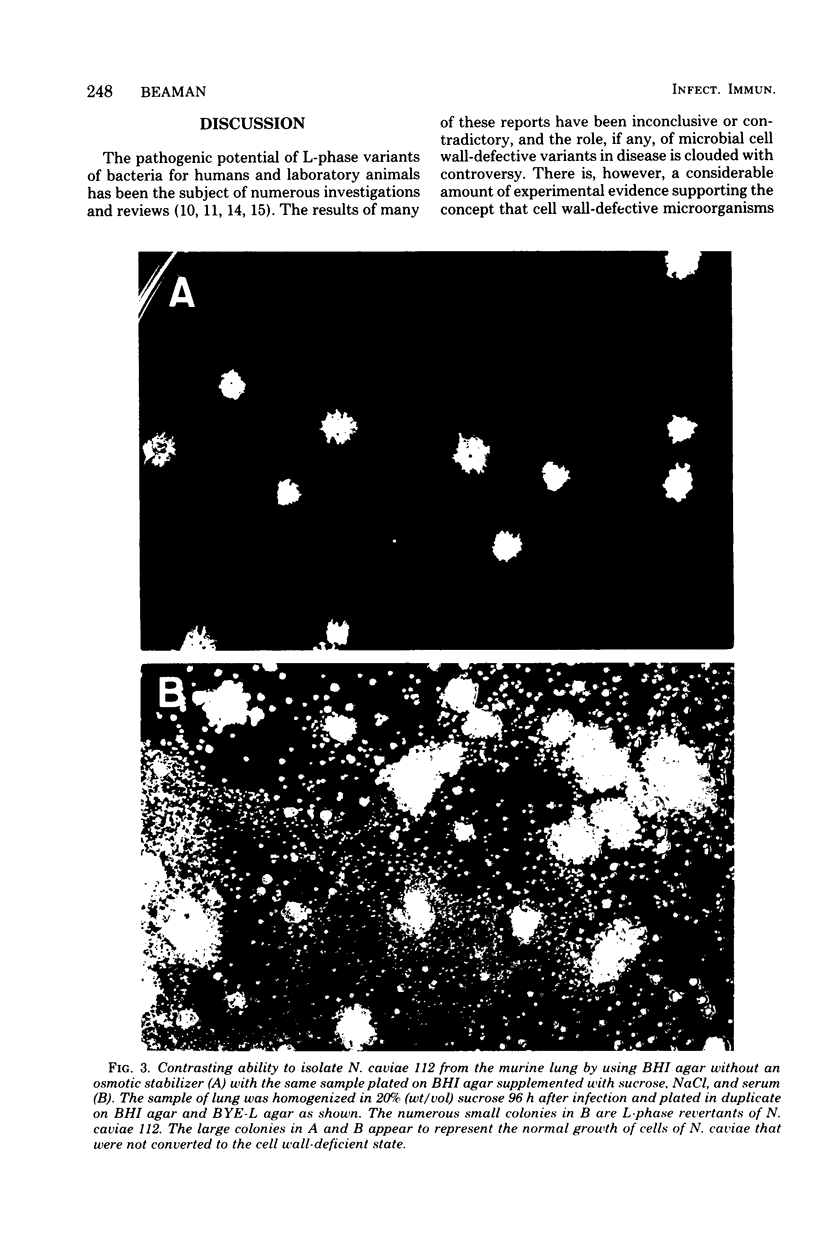
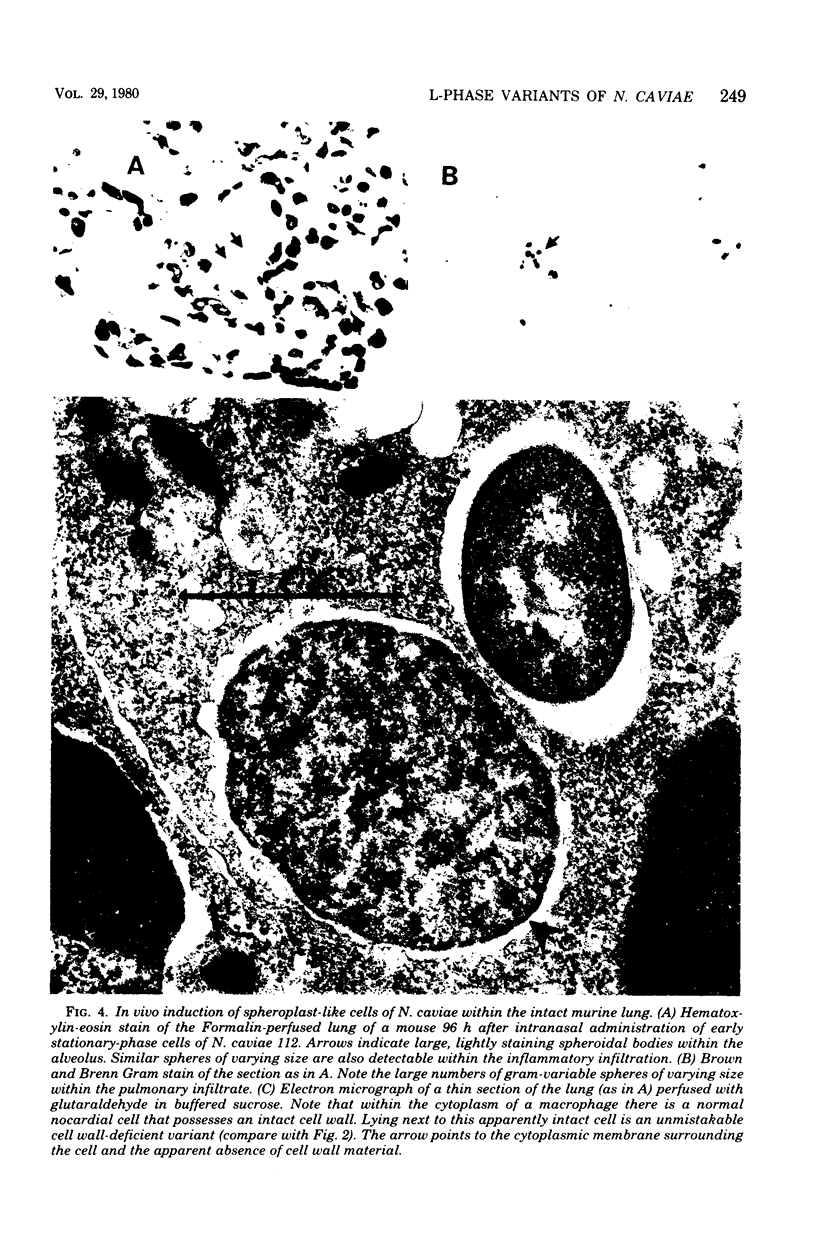
The data presented show that cells of Nocardia caviae 112 were converted to cell wall-deficient microbial variants within the intact murine lung after intranasal administration. At the time that these L-phase variants were recovered in large numbers from the lung, there was a correspondingly enhanced inflammation leading to alveolar consolidation and animal death. During the peak of this response (at 1 week after infection), normal nocardial cells were neither isolated from nor seen within the lung. It is suggested that the conversion of these normal nocardial cells to their L-phase variant leads to this extensive pulmonary damage. Furthermore, the L-phase organisms appear to play an active role in this pathological effect since introduction of similar amounts of killed nocardial cells into the lungs of the mice failed to produce a similar response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman B. L., Burnside J., Edwards B., Causey W. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976 Sep;134(3):286–289. doi: 10.1093/infdis/134.3.286. [DOI] [PubMed] [Google Scholar]
- Beaman B. L., Goldstein E., Gershwin M. E., Maslan S., Lippert W. Lung response to congenitally athymic (nude), heterozygous, and Swiss Webster mice to aerogenic and intranasal infection by Nocardia asteroides. Infect Immun. 1978 Dec;22(3):867–877. doi: 10.1128/iai.22.3.867-877.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Maslan S. Virulence of Nocardia asteroides during its growth cycle. Infect Immun. 1978 Apr;20(1):290–295. doi: 10.1128/iai.20.1.290-295.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Smathers M. Interaction of Nocardia asteroides with cultured rabbit alveolar macrophages. Infect Immun. 1976 Apr;13(4):1126–1131. doi: 10.1128/iai.13.4.1126-1131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois L., Beaman B. L. In vitro spheroplast and L-form induction within the pathogenic nocardiae. J Bacteriol. 1976 Jul;127(1):584–594. doi: 10.1128/jb.127.1.584-594.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois L., Beaman B. L. Probable L-forms of Nocardia asteroides induced in cultured mouse peritoneal macrophages. Infect Immun. 1974 Mar;9(3):576–590. doi: 10.1128/iai.9.3.576-590.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem A. M., Reidt W. A cell-wall deficient form of the pneumococcus in a case of pneumonia. Chest. 1972 Feb;61(2):200–202. doi: 10.1378/chest.61.2.200. [DOI] [PubMed] [Google Scholar]
- Charache P. Cell wall-defective bacterial variants in human disease. Ann N Y Acad Sci. 1970 Oct 30;174(2):903–911. doi: 10.1111/j.1749-6632.1970.tb45610.x. [DOI] [PubMed] [Google Scholar]
- Clasener H. Pathogenicity of the L-phase of bacteria. Annu Rev Microbiol. 1972;26:55–84. doi: 10.1146/annurev.mi.26.100172.000415. [DOI] [PubMed] [Google Scholar]
- Degré M. Phagocytic and bactericidal activities of peritoneal and alveolar macrophages from mice. J Med Microbiol. 1969 Aug;2(3):353–357. doi: 10.1099/00222615-2-3-353. [DOI] [PubMed] [Google Scholar]
- Feingold D. S. Biology and pathogenicity of microbial spheroplasts and l-forms. N Engl J Med. 1969 Nov 20;281(21):1159–1170. doi: 10.1056/NEJM196911202812106. [DOI] [PubMed] [Google Scholar]
- MYRVIK Q. N., LEAKE E. S., FARISS B. Lysozyme content of alveolar and peritoneal macrophages from the rabbit. J Immunol. 1961 Feb;86:133–136. [PubMed] [Google Scholar]
- McKay K. A., Abelseth M. K., Vandreumel A. A. Production of an enzootic-like pneumonia in pigs with "protoplasts" of Haemophilus parainfluenzae. Nature. 1966 Oct 22;212(5060):359–360. doi: 10.1038/212359a0. [DOI] [PubMed] [Google Scholar]
- Moscovic E. A. Sarcoidosis and mycobacterial L-forms. A critical reappraisal of pleomorphic chromogenic bodies (Hamazaki corpuscles) in lymph nodes. Pathol Annu. 1978;13(Pt 2):69–164. [PubMed] [Google Scholar]
- OSHIMA S., MYRVIK Q. N., LEAKE E. The demonstration of lysozyme as a dominant tuberculostatic factor in extracts of granulomatous lungs. Br J Exp Pathol. 1961 Apr;42:138–144. [PMC free article] [PubMed] [Google Scholar]
- Ratnam S., Chandrasekhar S. The pathogenicity of spheroplasts of Mycobacterium tuberculosis. Am Rev Respir Dis. 1976 Sep;114(3):549–554. doi: 10.1164/arrd.1976.114.3.549. [DOI] [PubMed] [Google Scholar]
- WITTLER R. G. The L-form of Haemophilus pertussis in the mouse. J Gen Microbiol. 1952 May;6(3-4):311–317. doi: 10.1099/00221287-6-3-4-311. [DOI] [PubMed] [Google Scholar]