Abstract
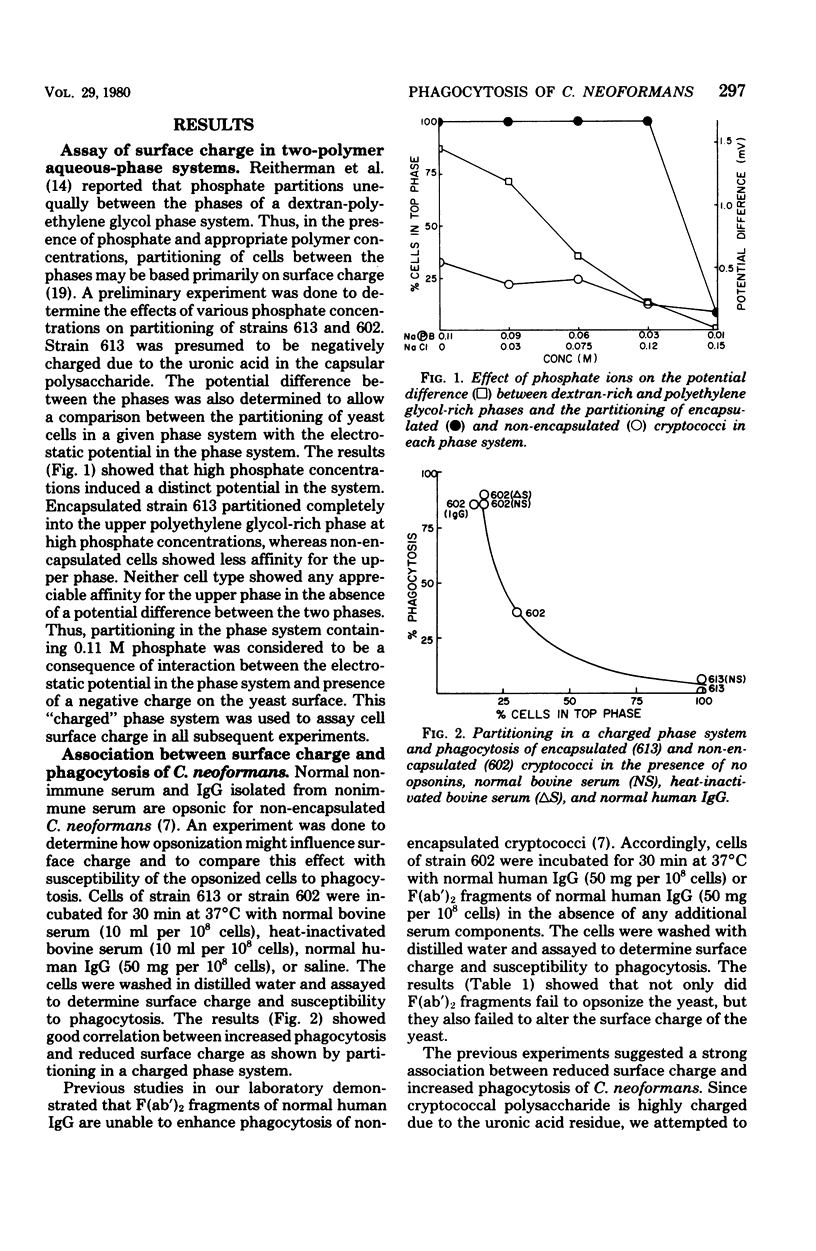
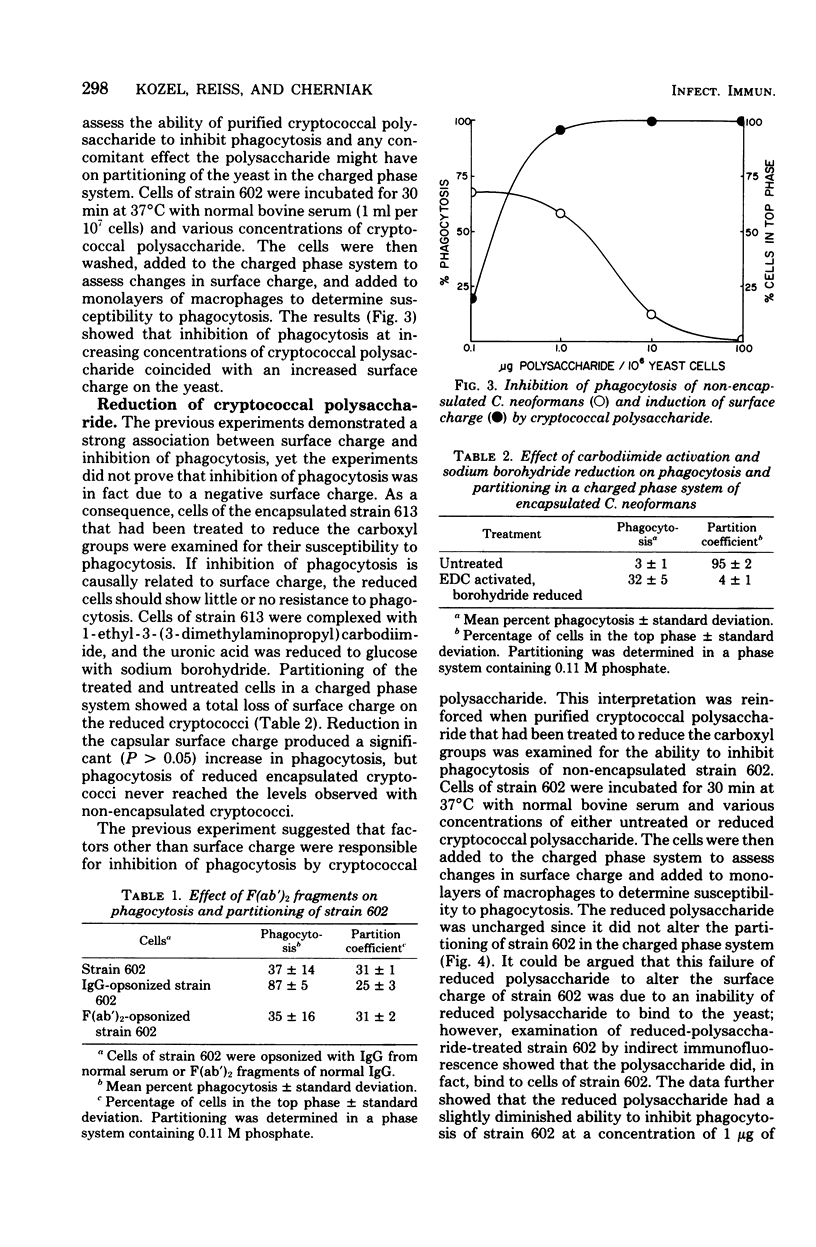
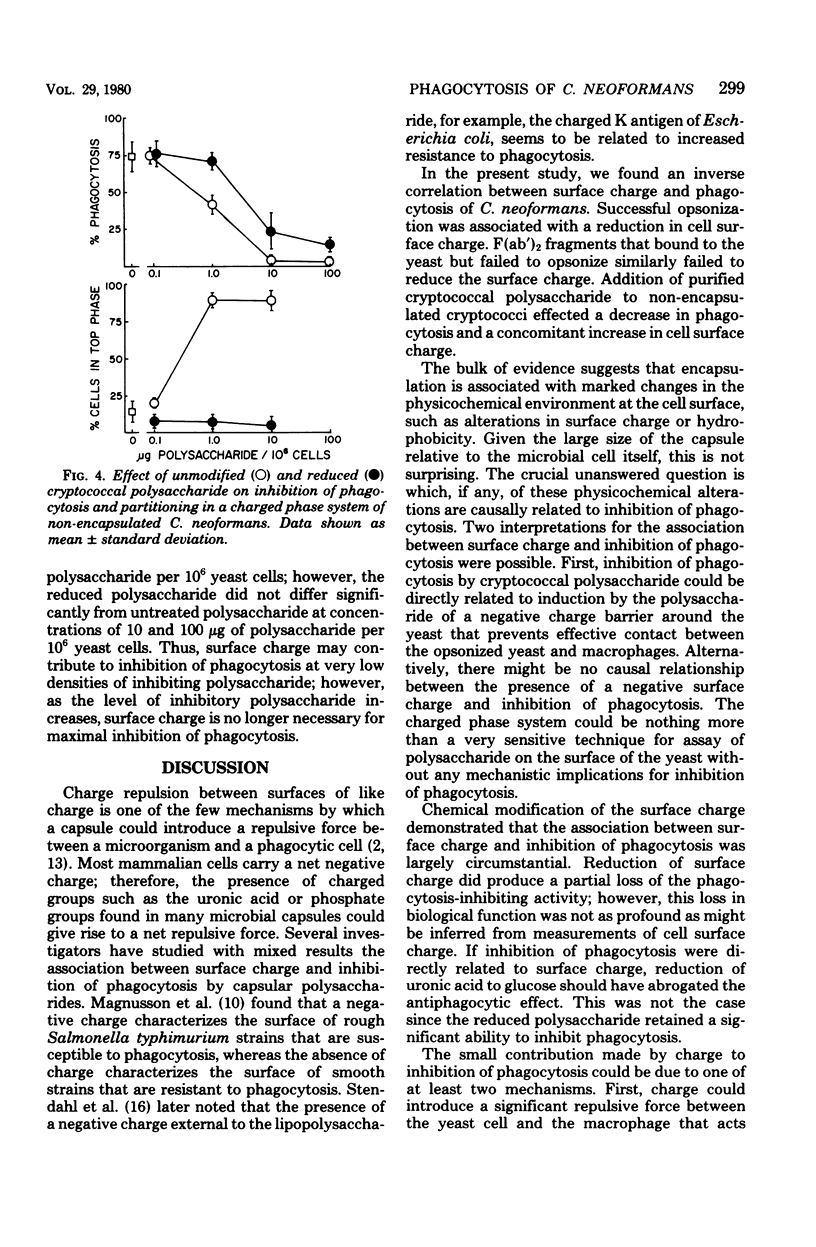
The mechanism by which capsular polysaccharides inhibit phagocytosis is not clearly understood. We investigated the association between a negative surface charge and inhibition of phagocytosis by the capsular polysaccharide of Cryptococcus neoformans. A two-polymer aqueous-phase system containing phosphate ions was used to assess surface charge. Opsonins such as normal bovine serum and normal human immunoglobulin G reduced the surface charge on non-encapsulated cryptococci and simultaneously enhanced phagocytosis. These same opsonins had no effect on phagocytosis or surface charge of encapsulated cryptococci. F (ab′)2 fragments of normal human immunoglobulin G neither enhanced phagocytosis nor altered the surface charge of non-encapsulated cryptococci. Addition of purified cryptococcal polysaccharide to non-encapsulated cells inhibited phagocytosis of the yeast and induced a strong negative charge at the yeast surface. Chemical modification to reduce the surface charge of either purified cryptococcal polysaccharide or intact encapsulated cryptococci produced a small loss of phagocytosis-inhibiting activity; however, all treated polysaccharide preparations retained a significant ability to inhibit phagocytosis of the yeast. These results indicated that the association between surface charge and inhibition of phagocytosis was largely circumstantial, and presence of a negative surface charge could not account for the powerful antiphagocytic action of cryptococcal polysaccharide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J. Nonencapsulated Variant of Cryptococcus neoformans I. Virulence Studies and Characterization of Soluble Polysaccharide. Infect Immun. 1971 Feb;3(2):287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Mastroianni R. P. Inhibition of phagocytosis by cryptococcal polysaccharide: dissociation of the attachment and ingestion phases of phagocytosis. Infect Immun. 1976 Jul;14(1):62–67. doi: 10.1128/iai.14.1.62-67.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., McGaw T. G. Opsonization of Cryptococcus neoformans by human immunoglobulin G: role of immunoglobulin G in phagocytosis by macrophages. Infect Immun. 1979 Jul;25(1):255–261. doi: 10.1128/iai.25.1.255-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R. Non-encapsulated variant of Cryptococcus neoformans. II. Surface receptors for cryptococcal polysaccharide and their role in inhibition of phagocytosis by polysaccharide. Infect Immun. 1977 Apr;16(1):99–106. doi: 10.1128/iai.16.1.99-106.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxalt K. A., Kozel T. R. Chemotaxigenesis and activation of the alternative complement pathway by encapsulated and non-encapsulated Cryptococcus neoformans. Infect Immun. 1979 Nov;26(2):435–440. doi: 10.1128/iai.26.2.435-440.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson K. E., Stendahl O., Tagesson C., Edebo L., Johansson G. The tendency of smooth and rough Salmonella typhimurium bacteria and lipopolysaccharide to hydrophobic and ionic interaction, as studied in aqueous polymer two-phase systems. Acta Pathol Microbiol Scand B. 1977 Jun;85(3):212–218. doi: 10.1111/j.1699-0463.1977.tb01698.x. [DOI] [PubMed] [Google Scholar]
- McGaw T. G., Kozel T. R. Opsonization of Cryptococcus neoformans by human immunoglobulin G: masking of immunoglobulin G by cryptococcal polysaccharide. Infect Immun. 1979 Jul;25(1):262–267. doi: 10.1128/iai.25.1.262-267.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagura H., Asai J., Katsumata Y., Kojima K. Role of electric surface charge of cell membrane in phagocytosis. Acta Pathol Jpn. 1973 May;23(2):279–290. doi: 10.1111/j.1440-1827.1973.tb00792.x. [DOI] [PubMed] [Google Scholar]
- PETHICA B. A. The physical chemistry of cell adhesion. Exp Cell Res. 1961;Suppl 8:123–140. doi: 10.1016/0014-4827(61)90344-5. [DOI] [PubMed] [Google Scholar]
- Reitherman R., Flanagan S. D., Barondes S. H. Electromotive phenomena in partition of erythrocytes in aqueous polymer two phase systems. Biochim Biophys Acta. 1973 Feb 28;297(2):193–202. doi: 10.1016/0304-4165(73)90065-2. [DOI] [PubMed] [Google Scholar]
- SPIEGELBERG H. L., WEIGLE W. O. THE CATABOLISM OF HOMOLOGOUS AND HETEROLOGOUS 7S GAMMA GLOBULIN FRAGMENTS. J Exp Med. 1965 Mar 1;121:323–338. doi: 10.1084/jem.121.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O., Normann B., Edebo L. Influence of O and K antigens on the surface properties of Escherichia coli in relation to phagocytosis. Acta Pathol Microbiol Scand B. 1979 Apr;87B(2):85–91. doi: 10.1111/j.1699-0463.1979.tb02408.x. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W. Structure of Klebsiella aerogenes type 8 polysaccharide. Biochemistry. 1970 May 12;9(10):2180–2185. doi: 10.1021/bi00812a022. [DOI] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- Wilkinson B. J., Peterson P. K., Quie P. G. Cryptic peptidoglycan and the antiphagocytic effect of the Staphylococcus aureus capsule: model for the antiphagocytic effect of bacterial cell surface polymers. Infect Immun. 1979 Feb;23(2):502–508. doi: 10.1128/iai.23.2.502-508.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


