Abstract
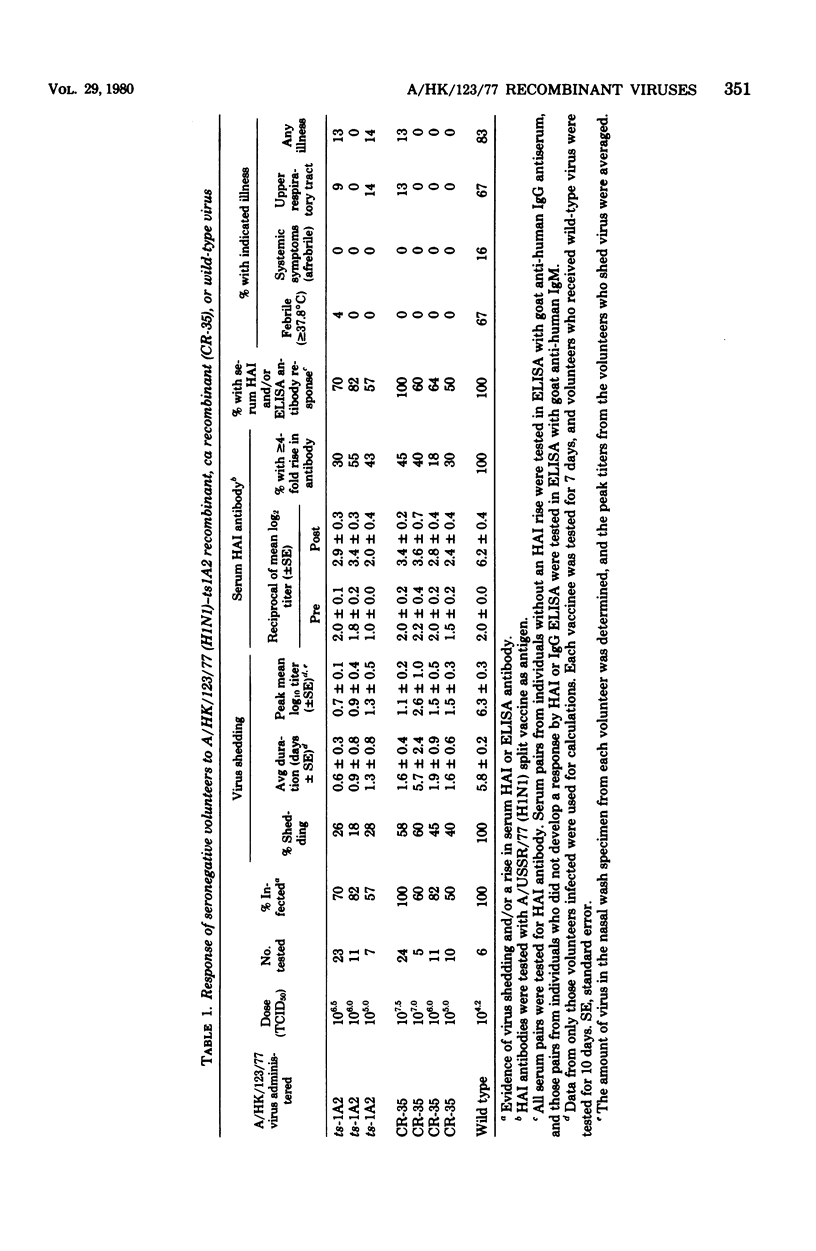
Two attenuated influenza A donor viruses, the A/Udorn/72 ts-1A2 and the A/Ann Arbor/6/60 cold-adapted (ca) viruses, are being evaluated for their ability to reproducibly attenuate each new variant of influenza A virus to a specific and desired level by the transfer of one or more attenuating genes. Each of these donor viruses has been able to attenuate influenza A viruses belonging to the H3N2 subtype by the transfer of one or more attenuating genes. To determine whether these two donor viruses could attenuate a wild-type virus that belonged to a different influenza A subtype, ts-1A2 and ca recombinants of a wild-type virus representative of the A/USSR/77 (H1N1) Russian influenza strain were prepared and evaluated in adult doubly seronegative volunteers at several doses. The recombinants derived from both donor viruses were attenuated for the doubly seronegative adults. Less than 5% of infected vaccinees developed a febrile or systemic reaction, whereas five of six recipients of wild-type virus developed such a response. The 50% human infectious dose (HID50) for each recombinant was approximately 105.0 50% tissue culture infective doses. The virus shed by the ts-1A2 and ca vaccinees retained the ts or ca phenotype, or both. This occurred despite replication of the recombinant viruses for up to 9 days. No evidence for transmission of the ca or ts-1A2 recombinant virus to controls was observed. A serum hemagglutination inhibition response was detected in less than 50% of the infected vaccinees. However, with the more sensitive enzyme-linked immunosorbent assay, a serological response was detected in 100% of the ca vaccinees given 300 HID50 and approximately 70% of ca or ts vaccinees who received 10 to 32 HID50 of virus. These results indicate that the recombinants derived from both donor viruses were satisfactorily attenuated and were stable genetically after replication in doubly seronegative adults although they induced a lower serum hemagglutination inhibition response than that found previously for H3N2 ts and ca recombinants.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox N. J., Maassab H. F., Kendal A. P. Comparative studies of wild-type and cold-mutant (temperature-sensitive) influenza viruses: nonrandom reassortment of genes during preparation of live virus vaccine candidates by recombination at 25 degrees between recent H3N2 and H1N1 epidemic strains and cold-adapted A/An Arbor/6/60. Virology. 1979 Aug;97(1):190–194. doi: 10.1016/0042-6822(79)90386-6. [DOI] [PubMed] [Google Scholar]
- Davenport F. M., Hennessy A. V., Maassab H. F., Minuse E., Clark L. C., Abrams G. D., Mitchell J. R. Pilot studies on recombinant cold-adapted live type A and B influenza virus vaccines. J Infect Dis. 1977 Jul;136(1):17–25. doi: 10.1093/infdis/136.1.17. [DOI] [PubMed] [Google Scholar]
- Gregg M. B., Hinman A. R., Craven R. B. The Russian flu. Its history and implications for this year's influenza season. JAMA. 1978 Nov 17;240(21):2260–2263. doi: 10.1001/jama.240.21.2260. [DOI] [PubMed] [Google Scholar]
- Hrabar A., Vodopija I., André F. E., Mitchell J. R., Maassab H. F., Hennessy A. V., Davenport F. M. A placebo-controlled dose-response study of the reactogenicity and immunogenicity of a cold-adapted recombinant A/Victoria/3/75 (H3N2) live influenza virus candidate vaccine in healthy volunteers. Dev Biol Stand. 1977 Jun 1;39:53–60. [PubMed] [Google Scholar]
- ISAACS A., GLEDHILL A. W., ANDREWES C. H. Influenza A viruses; laboratory studies, with special reference to European outbreak of 1950-1. Bull World Health Organ. 1952;6(3):287–315. [PMC free article] [PubMed] [Google Scholar]
- Kitayama T., Togo Y., Hornick R. B., Friedwald W. T. Low-Temperature-Adapted Influenza A2/AA/6/60 Virus Vaccine in Man. Infect Immun. 1973 Jan;7(1):119–122. doi: 10.1128/iai.7.1.119-122.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maassab H. F. Adaptation and growth characteristics of influenza virus at 25 degrees c. Nature. 1967 Feb 11;213(5076):612–614. doi: 10.1038/213612a0. [DOI] [PubMed] [Google Scholar]
- Maassab H. F., Francis T., Jr, Davenport F. M., Hennessy A. V., Minuse E., Anderson G. Laboratory and clinical characteristics of attenuated strains of influenza virus. Bull World Health Organ. 1969;41(3):589–594. [PMC free article] [PubMed] [Google Scholar]
- Massicot J. G., Murphy B. R., Thierry F., Markoff L., Huang K. Y., Chanock R. M. Temperature-sensitive mutants of influenza virus. Identification of the loci of the two ts lesions in the Udorn-ts-1A2 donor virus and the correlation of the presence of these two ts lesions with a predictable level of attenuation. Virology. 1980 Feb;101(1):242–249. doi: 10.1016/0042-6822(80)90499-7. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Chanock R. M., Levine M. M., van Blerk G. A., Berquist E. J., Douglas R. G., Betts R. F., Couch R. B., Cate T. R., Jr Temperature-sensitive mutants of influenza A virus: evaluation of the A/Victoria/75-ts-1A2 temperature-sensitive recombinant virus in seronegative adult volunteers. Infect Immun. 1979 Feb;23(2):249–252. doi: 10.1128/iai.23.2.249-252.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Holley H. P., Jr, Berquist E. J., Levine M. M., Spring S. B., Maassab H. F., Kendal A. P., Chanock R. M. Cold-adapted variants of influenza A virus: evaluation in adult seronegative volunteers of A/Scotland/840/74 and A/Victoria/3/75 cold-adapted recombinants derived from the cold-adapted A/Ann Arbor/6/60 strain. Infect Immun. 1979 Feb;23(2):253–259. doi: 10.1128/iai.23.2.253-259.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Markoff L. J., Chanock R. M., Spring S. B., Maassab H. F., Kendal A. P., Cox N. J., Levine M. M., Douglas R. G., Jr, Betts R. F. Genetic approaches to attenuation of influenza A viruses for man. Philos Trans R Soc Lond B Biol Sci. 1980 Feb 25;288(1029):401–415. doi: 10.1098/rstb.1980.0017. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Markoff L. J., Hosier N. T., Rusten H. M., Chanock R. M., Kendal A. P., Douglas R. G., Betts R. F., Cate T. R., Jr, Couch R. B. Temperature-sensitive mutants of influenza A virus: evaluation of A/Victoria/3/75-ts-1[E] recombinant viruses in volunteers. Infect Immun. 1978 Jun;20(3):671–677. doi: 10.1128/iai.20.3.671-677.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Tierney E. L., Barbour B. A., Yolken R. H., Alling D. W., Holley H. P., Jr, Mayner R. E., Chanock R. M. Use of the enzyme-linked immunosorbent assay to detect serum antibody responses of volunteers who received attenuated influenza A virus vaccines. Infect Immun. 1980 Aug;29(2):342–347. doi: 10.1128/iai.29.2.342-347.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Wood F. T., Massicot J. G., Chanock R. M. Temperature-sensitive mutants of influenza virus. XVI. Transfer of the two ts lesions present in the Udorn/72-ts-1A2 donor virus to the Victoria/3/75 wild-type virus. Virology. 1978 Jul 15;88(2):244–251. doi: 10.1016/0042-6822(78)90281-7. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Wood F. T., Massicot J. G., Spring S. B., Chanock R. M. Temperature-sensitive mutants of influenza virus. XV. The genetic and biological characterization of a recombinant influenza virus containing two ts lesions produced by mating two complementing, single lesion ts mutants. Virology. 1978 Jul 15;88(2):231–243. doi: 10.1016/0042-6822(78)90280-5. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Murphy B. R., Chanock R. M., Gwaltney J. M., Jr, Douglas R. G., Betts R. F., Blacklow N. R., Rose F. B., Parrino T. A., Levine M. M. Temperature-sensitive mutants of influenza A virus. XII. Safety, antigenicity, transmissibility, and efficacy of influenza A/Udorn/72-ts-1[E] recombinant viruses in human adults. J Infect Dis. 1976 Dec;134(6):585–594. [PubMed] [Google Scholar]
- Spring S. B., Maassab H. F., Kendal A. P., Murphy B. R., Chanock R. M. Cold adapted variants of influenza A. II. Comparison of the genetic and biological properties of ts mutants and recombinants of the cold adapted A/AA/6/60 strain. Arch Virol. 1977;55(3):233–246. doi: 10.1007/BF01319909. [DOI] [PubMed] [Google Scholar]
- Spring S. B., Maassab H. F., Kendal A. P., Murphy B. R., Chanock R. M. Cold-adapted variants of influenza virus A. I. Comparison of the genetic properties of ts mutants and five cold-adapted variants of influenza virus A. Virology. 1977 Mar;77(1):337–343. doi: 10.1016/0042-6822(77)90430-5. [DOI] [PubMed] [Google Scholar]
- Spring S. B., Nusinoff S. R., Mills J., Richman D. D., Tierney E. L., Murphy B. R., Chanock R. M. Temperature-sensitive mutants of influenza virus. VI. Transfer of TS lesions from the Asian subtype of influenza A virus (H2N2) to the Hong Kong subtype (H3N2). Virology. 1975 Aug;66(2):522–532. doi: 10.1016/0042-6822(75)90224-x. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Sell S. H., Shinozaki T., Thompson J., Karzon D. T. Safety and antigenicity of influenza A/Hong Kong/68-ts-1 (E) (H3N2). J Pediatr. 1975 Dec;87(6 Pt 2):1109–1116. doi: 10.1016/s0022-3476(75)80123-5. [DOI] [PubMed] [Google Scholar]


