Abstract
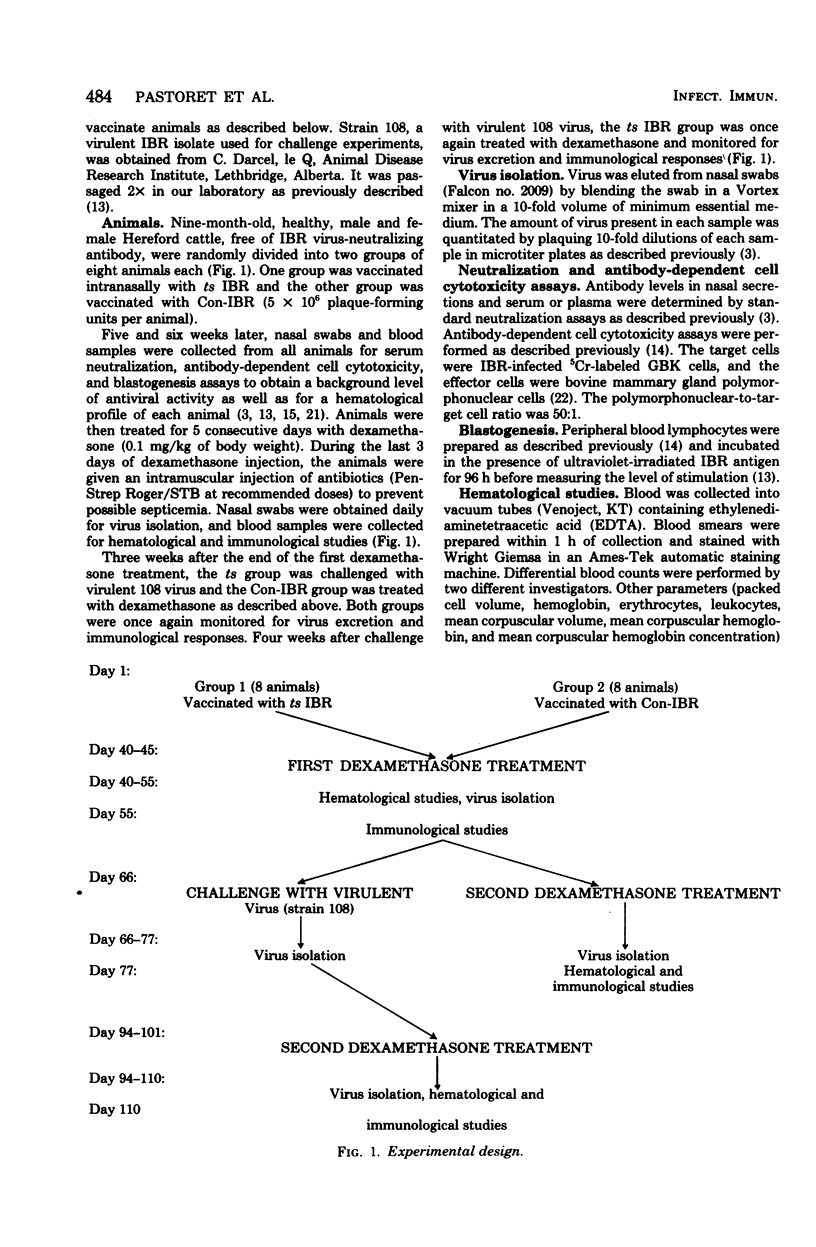
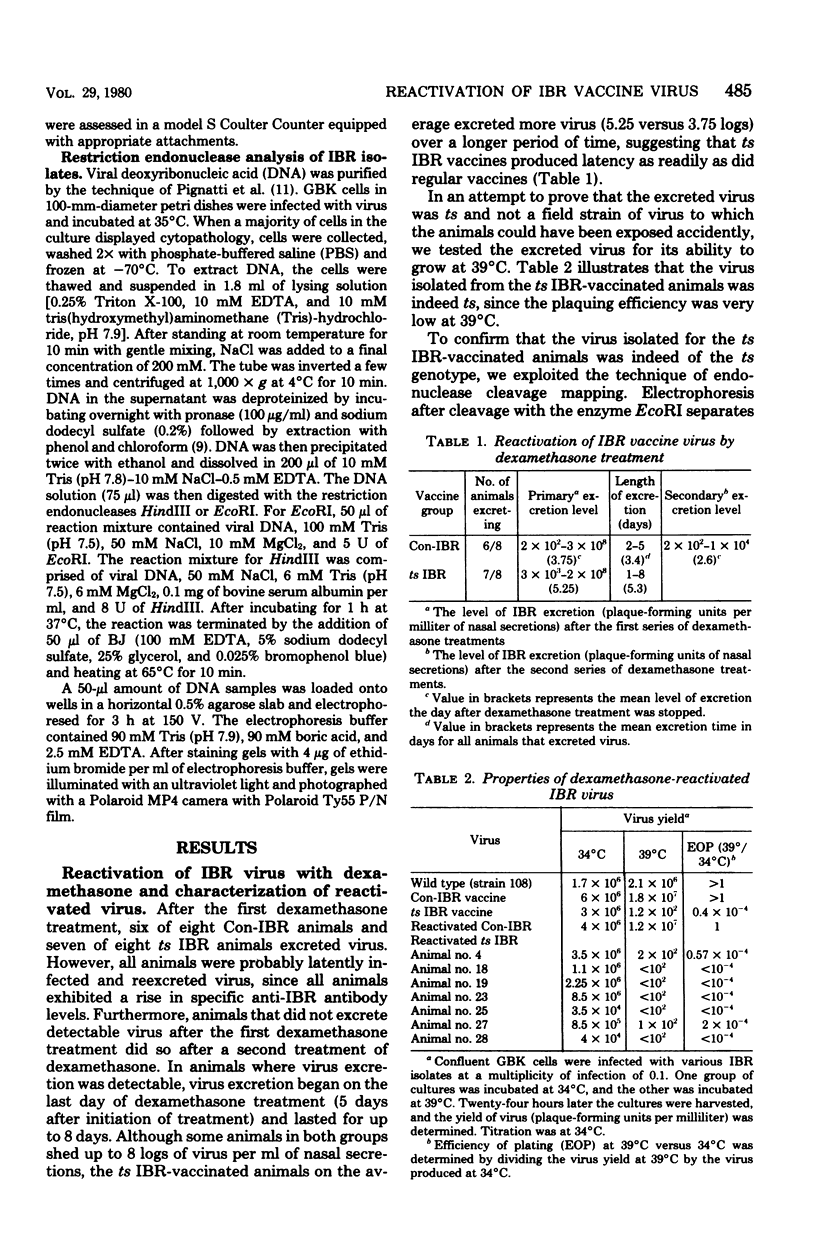
Latent infections by a temperature-sensitive (ts) infectious bovine rhinotracheitis virus vaccines was produced as frequently as by non-ts vaccine virus. Thus virus could be reactivated in seven of eight ts vaccinates and six of eight non-ts vaccinates after dexamethasone treatment. Virus excretion could be detectable for 1 to 8 days at a level of 2 x 10(6) to 3 x 10(8) plaque-forming units per ml of nasal secretions. The reactivated virus was shown to be the same as the original virus used for vaccination by its inability to grow at the restrictive temperature (39 degrees C) as well as by its restriction endonuclease cleavage pattern.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., Rouse B. T. Immune control of herpesvirus latency. Can J Microbiol. 1979 Mar;25(3):267–274. doi: 10.1139/m79-043. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., Rouse B. T. Immune interferon production by lymphoid cells: role in the inhibition of herpesviruses. Infect Immun. 1976 Jun;13(6):1567–1578. doi: 10.1128/iai.13.6.1567-1578.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiuk L. A., Wardley R. C., Rouse B. T. Defense mechanisms against bovine herpesvirus: relationship of virus-host cell events to susceptibility to antibody-complement cell lysis. Infect Immun. 1975 Nov;12(5):958–963. doi: 10.1128/iai.12.5.958-963.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. H., Carmichael L. E. Role of cell-mediated immunity in the recovery of cattle from primary and recurrent infections with infectious bovine rhinotracheitis virus. Infect Immun. 1973 Oct;8(4):510–518. doi: 10.1128/iai.8.4.510-518.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J. C., Marsden H. S., Cook M. L., Stevens J. G. Acute infection of differentiated neuroblastoma cells by latency-positive and latency-negative herpes simplex virus ts mutants. Virology. 1979 Apr 30;94(2):430–441. doi: 10.1016/0042-6822(79)90473-2. [DOI] [PubMed] [Google Scholar]
- Lehner T., Wilton J. M., Shillitoe E. J. Immunological basis for latency, recurrences and putative oncogenicity of herpes simplex virus. Lancet. 1975 Jul 12;2(7924):60–62. doi: 10.1016/s0140-6736(75)90499-7. [DOI] [PubMed] [Google Scholar]
- Lofgren K. W., Stevens J. G., Marsden H. S., Subak-Sharpe J. H. Temperature-sensitive mutants of herpes simplex virus differ in the capacity to establish latent infections in mice. Virology. 1977 Jan;76(1):440–443. doi: 10.1016/0042-6822(77)90319-1. [DOI] [PubMed] [Google Scholar]
- Misra V., Muller M. T., Chantler J. K., Hudson J. B. Regulation of murine cytomegalovirus gene expression. I. Transcription during productive infection. J Virol. 1978 Aug;27(2):263–268. doi: 10.1128/jvi.27.2.263-268.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter M. F., Docherty J. J. Steroid hormone alteration of herpes simplex virus type 1 replication. J Med Virol. 1978;2(3):247–252. doi: 10.1002/jmv.1890020308. [DOI] [PubMed] [Google Scholar]
- Pignatti P. F., Cassai E., Meneguzzi G., Chenciner N., Milanesi G. Herpes simplex virus DNA isolation from infected cells with a novel procedure. Virology. 1979 Feb;93(1):260–264. doi: 10.1016/0042-6822(79)90295-2. [DOI] [PubMed] [Google Scholar]
- Plowright W. Vaccination against diseases associated with herpesvirus infections in animals: a review. IARC Sci Publ. 1978;(24 Pt 2):965–980. [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Host defense mechanisms against infectious bovine rhinotracheitis virus: in vitro stimulation of sensitized lymphocytes by virus antigen. Infect Immun. 1974 Oct;10(4):681–687. doi: 10.1128/iai.10.4.681-687.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Host responses to infectious bovine rhinotracheitis virus. III. Isolation and immunologic activities of bovine T lymphocytes. J Immunol. 1974 Nov;113(5):1391–1398. [PubMed] [Google Scholar]
- Rouse B. T., Wardley R. C., Babiuk L. A. Antibody-dependent cell-mediated cytotoxicity in cows: comparison of effector cell activity against heterologous erthrocyte and herpesvirus-infected bovine target cells. Infect Immun. 1976 May;13(5):1433–1441. doi: 10.1128/iai.13.5.1433-1441.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffy B. E., Davies D. H. Reactivation of a bovine herpesvirus after corticosteroid treatment. Proc Soc Exp Biol Med. 1972 Jul;140(3):974–976. doi: 10.3181/00379727-140-36592. [DOI] [PubMed] [Google Scholar]
- Skare J., Summers W. C. Structure and function of herpesvirus genomes. II. EcoRl, Sbal, and HindIII endonuclease cleavage sites on herpes simplex virus. Virology. 1977 Feb;76(2):581–595. doi: 10.1016/0042-6822(77)90240-9. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Maintenance of latent herpetic infection: an apparent role for anti-viral IgG. J Immunol. 1974 Dec;113(6):1685–1693. [PubMed] [Google Scholar]
- Stevens J. G. Latent characteristics of selected herpesviruses. Adv Cancer Res. 1978;26:227–256. doi: 10.1016/s0065-230x(08)60089-5. [DOI] [PubMed] [Google Scholar]
- Wardley R. C., Babiuk L. A., Rouse B. T. Polymorph-mediated antibody-dependent cytoxicity--modulation of activity by drugs and immune interferon. Can J Microbiol. 1976 Sep;22(9):1222–1228. doi: 10.1139/m76-181. [DOI] [PubMed] [Google Scholar]
- Wardley R. C., Rouse B. T., Babiuk L. A. The mammary gland of the ox: a convenient source for the repeated collection of neutrophils and macrophages. J Reticuloendothel Soc. 1976 Jan;19(1):29–36. [PubMed] [Google Scholar]
- Zygraich N., Vascoboinic E., Huygelen C. Replication of temperature sensitive mutant of infectious bovine rhinotracheitis virus in the tissues of inoculated calves. Zentralbl Veterinarmed B. 1974 Mar;21(3):138–144. [PubMed] [Google Scholar]



