Abstract
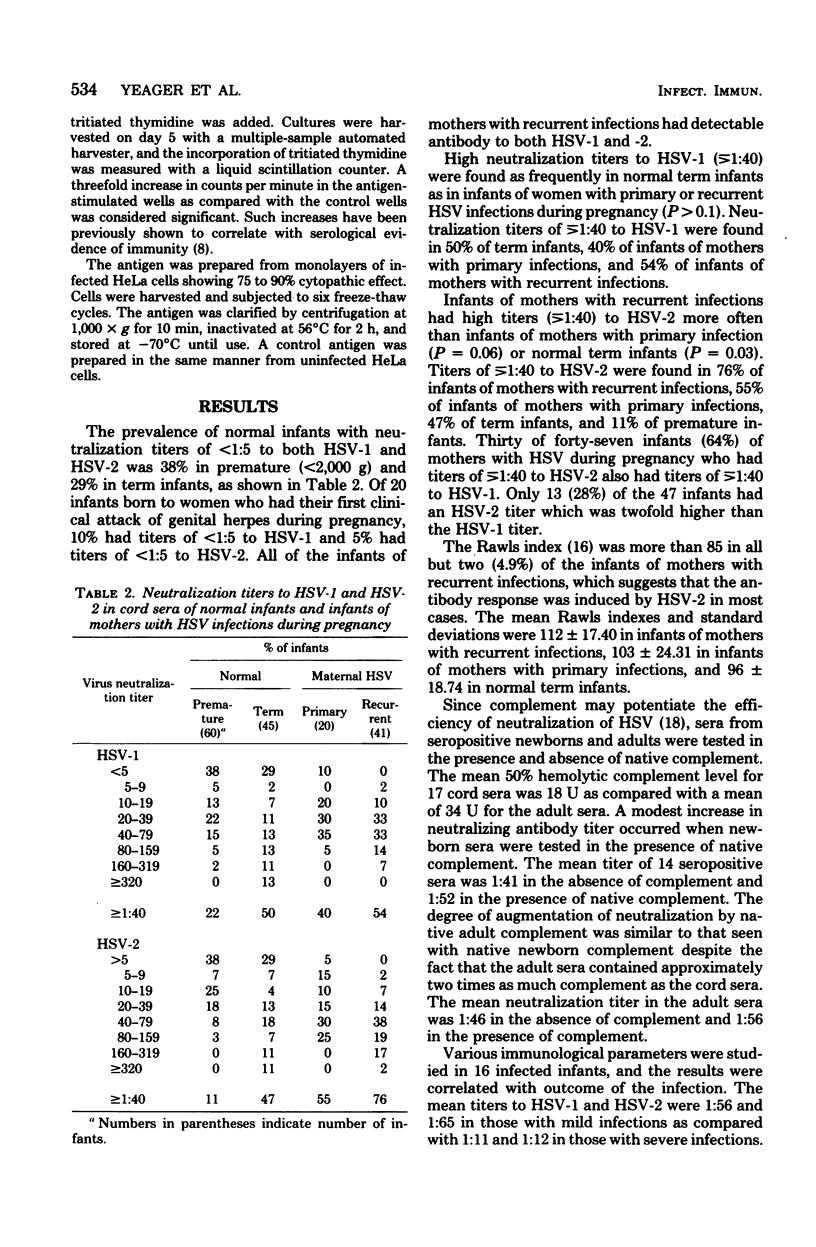
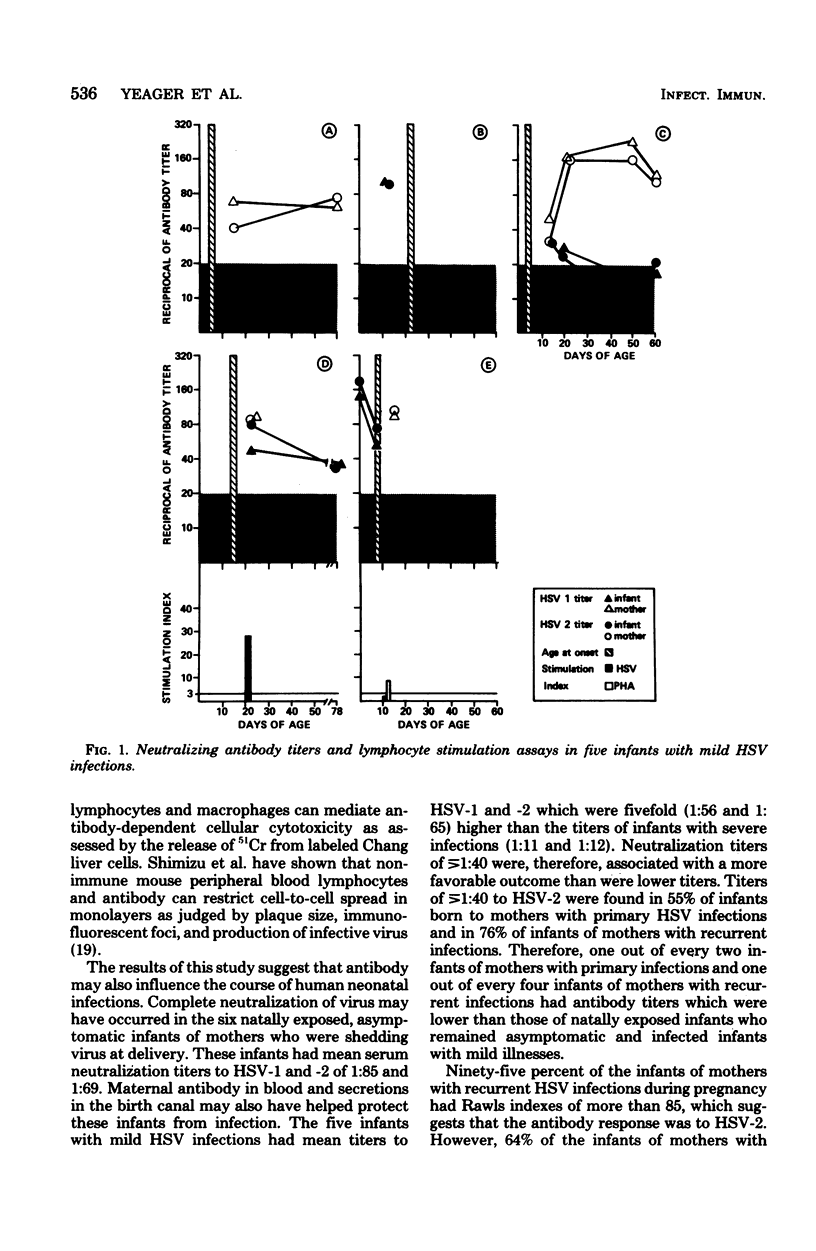
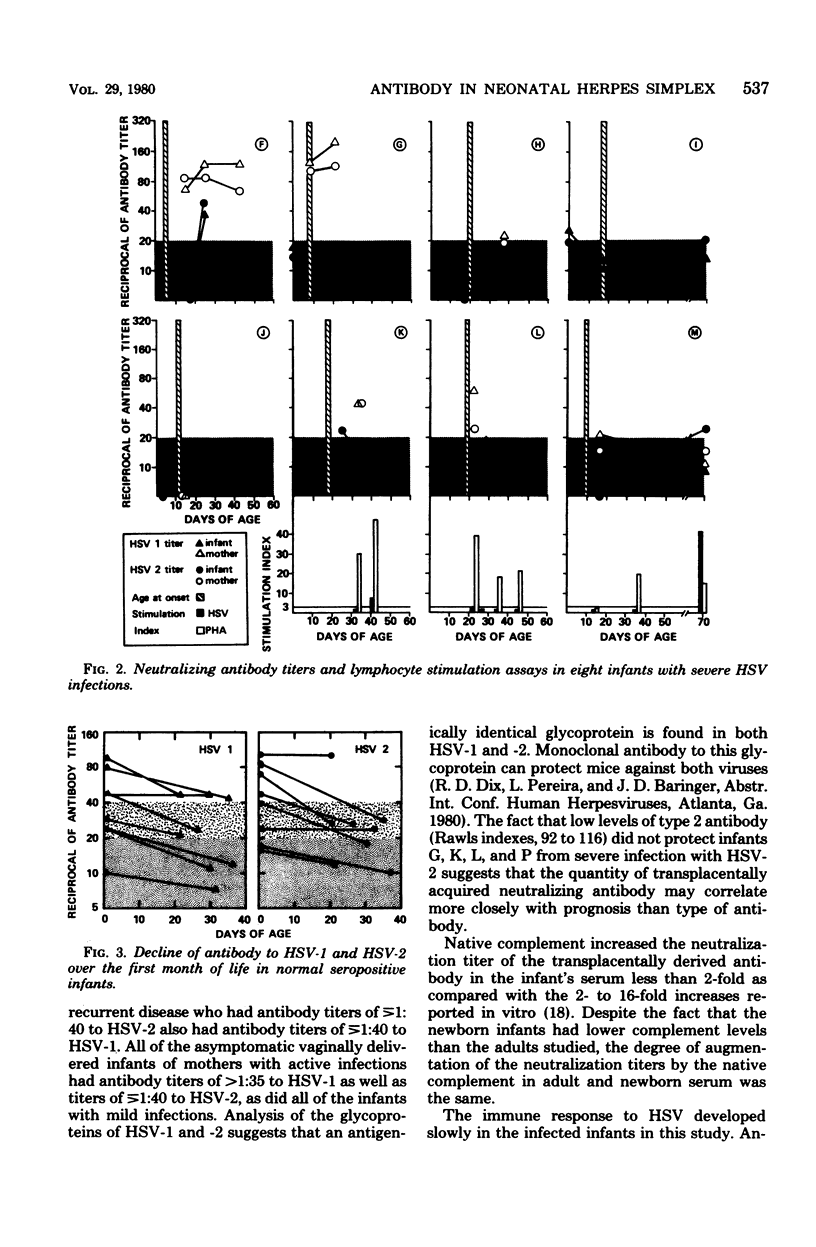
Neutralizing antibody titers to herpes simplex virus type 1 (HSV-1) and HSV-2 were measured at birth in normal infants and uninfected infants of mothers with genital HSV infections during pregnancy and at the onset of infection in 5 infants with mild infections and 11 infants with severe infections. Thirty-eight percent of premature and 29% of term infants had neutralization titers of <1:5. High titers ([unk]1:40) were found in 55% of infants of mothers with primary infections during pregnancy and in 76% of infants of mothers with recurrent infections. The mean titers to HSV-1 and -2 in 5 infected infants with mild infections were 1:56 and 1:65 at the time of onset of infection, whereas the mean titers in 11 infants with severe infections were 1:11 and 1:12. Six natally exposed infants who remained asymptomatic were also studied and had a mean titer to HSV-1 of 1:85 and to HSV-2 of 1:69. Therefore, infants with high titers of transplacentally derived antibody had a more favorable outcome than infants with lower titers. Ninety-five percent of the infants of mothers with recurrent infections had a Rawls index of more than 85, suggesting that the antibody response was to HSV-2. However, low levels of antibody with this type specificity failed to protect four infants from infection with HSV-2. Augmentation of the neutralization titer to HSV-2 by the amount of complement present in cord serum was less than twofold. The study suggests that the quantity of antibody derived transplacentally affects the outcome of infection after natal exposure to herpes simplex virus. Complete neutralization of virus by antibody may occur in some infants, and prolongation of the incubation period and modification of the infection may occur in others.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams H. G., Benson E. A., Alexander E. R., Vontver L. A., Remington M. A., Holmes K. K. Genital herpetic infection in men and women: clinical course and effect of topical application of adenine arabinoside. J Infect Dis. 1976 Jun;133 (Suppl):A151–A159. doi: 10.1093/infdis/133.supplement_2.a151. [DOI] [PubMed] [Google Scholar]
- Baron S., Worthington M. G., Williams J., Gaines J. W. Postexposure serum prophylaxis of neonatal herpes simplex virus infection of mice. Nature. 1976 Jun 10;261(5560):505–506. doi: 10.1038/261505a0. [DOI] [PubMed] [Google Scholar]
- Brown Z. A., Kern E. R., Spruance S. L., Overall J. C., Jr Clinical and virologic course of herpes simplex genitalis. West J Med. 1979 May;130(5):414–421. [PMC free article] [PubMed] [Google Scholar]
- Ch'ien L. T., Whitley R. J., Nahmias A. J., Lewin E. B., Linnemann C. C., Jr, Frenkel L. D., Bellanti J. A., Buchanan R. A., Alford D. A., Jr Antiviral chemotherapy and neonatal herpes simplex virus infecition: a pilot study--experience with adenine arabinoside (ARA-A). Pediatrics. 1975 May;55(5):678–685. [PubMed] [Google Scholar]
- Cho C. T., Feng K. K., Brahmacupta N. Synergistic antiviral effects of adenine arabinoside and humoral antibodies in experimental encephalitis due to Herpesvirus hominis. J Infect Dis. 1976 Feb;133(2):157–167. doi: 10.1093/infdis/133.2.157. [DOI] [PubMed] [Google Scholar]
- Corey L., Reeves W. C., Holmes K. K. Cellular immune response in genital herpes simplex virus infection. N Engl J Med. 1978 Nov 2;299(18):986–991. doi: 10.1056/NEJM197811022991805. [DOI] [PubMed] [Google Scholar]
- Gardner H. L., Kaufman R. H. Herpes genitalis: clinical features. Clin Obstet Gynecol. 1972 Dec;15(4):896–911. doi: 10.1097/00003081-197212000-00004. [DOI] [PubMed] [Google Scholar]
- Haahr S., Rasmussen L., Merigan T. C. Lymphocyte transformation and interferon production in human mononuclear cell microcultures for assay of cellular immunity to herpes simplex virus. Infect Immun. 1976 Jul;14(1):47–54. doi: 10.1128/iai.14.1.47-54.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S., Shaban S. S., Starr S. E., Wood P. A., Nahmias A. J. Human neonatal and maternal monocyte-macrophage and lymphocyte-mediated antibody-dependent cytotoxicity to cells infected with herpes simplex. J Pediatr. 1978 Aug;93(2):206–210. doi: 10.1016/s0022-3476(78)80497-1. [DOI] [PubMed] [Google Scholar]
- Lodmell D. L., Niwa A., Hayashi K., Notkins A. L. Prevention of cell-to-cell spread of herpes simplex virus by leukocytes. J Exp Med. 1973 Mar 1;137(3):706–720. doi: 10.1084/jem.137.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyet F., Samra D., Soneji A., Marks M. I. Passive immunization in experimental Herpesvirus hominis infection of newborn mice. Infect Immun. 1975 Dec;12(6):1258–1261. doi: 10.1128/iai.12.6.1258-1261.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias A. J., Josey W. E., Naib Z. M., Freeman M. G., Fernandez R. J., Wheeler J. H. Perinatal risk associated with maternal genital herpes simplex virus infection. Am J Obstet Gynecol. 1971 Jul 15;110(6):825–837. doi: 10.1016/0002-9378(71)90580-1. [DOI] [PubMed] [Google Scholar]
- Ng A. B., Reagan J. W., Yen S. S. Hepres genitalis; clinical and cytopathologic experience with 256 patients. Obstet Gynecol. 1970 Oct;36(4):645–651. [PubMed] [Google Scholar]
- Oakes J. E., Rosemond-Hornbeak H. Antibody-mediated recovery from subcutaneous herpes simplex virus type 2 infection. Infect Immun. 1978 Aug;21(2):489–495. doi: 10.1128/iai.21.2.489-495.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls W. E., Iwamoto K., Adam E., Melnick J. L. Measurement of antibodies to herpesvirus types 1 and 2 in human sera. J Immunol. 1970 Mar;104(3):599–606. [PubMed] [Google Scholar]
- Schmidt N. J., Forghani B., Lennette E. H. Type specificity of complement-requiring and immunoglobulin M neutralizing antibody in initial herpes simplex virus infections of humans. Infect Immun. 1975 Oct;12(4):728–732. doi: 10.1128/iai.12.4.728-732.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu F., Hanaumi K., Shimizu Y., Kumagai K. Antibody-dependent cellular protection against herpes simplex virus dissemination as revealed by viral plauqe and infectivity assays. Infect Immun. 1977 May;16(2):531–536. doi: 10.1128/iai.16.2.531-536.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S. L., Milgrom H., Wood P. A., Nahmias A. J. Antibody-dependent cellular cytotoxicity to target cells infected with herpes simplex viruses: functional adequacy in the neonate. Pediatrics. 1977 Jan;59(1):22–28. [PubMed] [Google Scholar]


