Abstract
Purpose
Platinum-based chemotherapy remains the standard treatment for advanced urothelial carcinoma by inducing DNA damage. We hypothesize that somatic alterations in DNA damage response and repair (DDR) genes are associated with improved sensitivity to platinum-based chemotherapy.
Experimental Design
Patients with diagnosis of locally advanced and metastatic urothelial carcinoma treated with platinum-based chemotherapy who had exon sequencing with MSK-IMPACT assay were identified. Patients were dichotomized based on presence/absence of alterations in a panel of 34 DDR genes. DDR alteration status was correlated with clinical outcomes and disease features.
Results
One hundred patients were identified, of which 47 harbored alterations in DDR genes. Patients with DDR alterations had improved progression-free survival (9.3 vs. 6.0 months, log rank p=0.007) and overall survival (23.7 vs. 13.0 months, log rank p=0.006). DDR alterations were also associated with higher number mutations and copy number alterations. A trend towards positive correlation between DDR status and nodal metastases and inverse correlation with visceral metastases were observed. Different DDR pathways also suggested variable impact on clinical outcomes.
Conclusion
Somatic DDR alteration is associated with improved clinical outcomes in platinum-treated patients with advanced urothelial carcinoma. Once validated, it can improve patient selection for clinical practice and future study enrollment.
Keywords: urothelial carcinoma, cisplatin, DNA damage response and repair, biomarkers, genomic
Introduction
Platinum-based chemotherapy regimens remain the standard of care for locally-advanced and metastatic urothelial carcinoma. Cisplatin-based chemotherapy has consistently been associated with response rates of 40–50% and median OS in the range of 14–15 months 1,2, while carboplatin-based regimens have similar response rates but median OS between 9 and 12 months.3-5 Efforts to improve outcomes, such as the addition of new agents or dose intensification, have not resulted in substantial improvements. Clinical variables such as the presence of visceral metastases and impaired performance status have been shown to define poor prognostic categories.6 Recently, next generation sequencing has revealed underlying biological drivers of carcinogenesis and disease progression for urothelial carcinoma.7-9 However, no clinically validated predictive marker for therapeutic efficacy exists for patients with metastatic urothelial carcinoma treated with platinum-based chemotherapy.
Platinum compounds exert cytotoxic effects by forming DNA adducts which interfere with DNA replication and consequent gene transcription. However, complex mechanisms are in place to detect DNA damage and initiate various repair pathways. Inactivating alterations of genes involved in DNA repair pathways are frequently observed in cancer and the presence of somatic alterations in these genes have been reported to correlate with improved pathologic response following neoadjuvant cisplatin-based chemotherapy and possibly overall survival in urothelial carcinoma.10-12
In this study, we sought to determine whether alterations of genes involved in DNA damage response and repair (DDR) are associated with improved sensitivity to platinum-based chemotherapy, as measured by objective response, progression free survival (PFS), and overall survival (OS) in patients with metastatic urothelial carcinoma.
Methods
Study Design and Patients
Between 10/2012 and 09/2015, next generation sequencing was performed on consecutive cases of bladder cancer. Patients were included in this study if they had histologically confirmed urothelial carcinoma, radiographic evidence of unresectable locally advanced or metastatic disease, receipt of platinum-based chemotherapy as first-line therapy in a non-curative setting, and sequencing performed on specimens prior to commencement of platinum chemotherapy. In addition, 10 specimens from DFCI with de-identified clinical data were included.
The primary objective was to examine the association between the presence of any DDR gene alteration and objective response, PFS and OS. We also explored the effect on these clinical endpoints by the number of individual DDR gene alterations in a given tumor identified on our institutional next generation sequencing platform and the number of DNA repair pathways involved on these endpoints. Thirdly, we assessed the association of DDR gene alterations with both total tumor mutation and copy number alteration burden.
Data Collection
Baseline patient and disease characteristics were extracted from institutional electronic health records. Variables collected include: gender, age, ECOG performance status, site of primary tumor, prior neoadjuvant or adjuvant platinum chemotherapy treatment, sites of metastatic disease, and treatment regimens received.
Tumor Sequencing
All protein-coding exons of 341 cancer-associated genes were sequenced using the Integrated Molecular Profiling of Actionable Cancer Targets (MSK-IMPACT) assay.13 Thirty-four genes within MSK-IMPACT were identified as DDR-related based on Pubmed searches and the NCBI Gene and Biosystems Databases (Supplemental table 1).
Determination of Deleterious Mutation Status
All loss of function alterations were considered deleterious, including nonsense mutations, frameshift or splice site alterations.
Two different approaches were employed to determine functional impact of missense mutations. The first was via in silico functional analysis using Polyphen-214 and MutationAssessor15 which employed various predictive features, alignment pipelines and method of classification to predict the functional impact of amino acid substitutions in proteins. Any missense mutations classified as “possibly damaging” or “probably damaging” in Polyphen2, or “medium” or “high” in MutationAssessor were considered deleterious.
The second method was previously described by Iyer et al 16 and involved manual review of identified missense mutations in Catalogue of Somatic Mutations in Cancer (COSMIC), recurrent hotspot mutations 17 and PubMed searches. Based on previous work 11, all ERCC2 missense mutations within or near the helicase domains were considered deleterious.
MSK-IMPACT Gene Panel as Surrogate of Total Mutational Burden
To demonstrate that mutation load estimates based on our MSK-IMPACT assay largely recapitulated what whole exome sequencing would have generated, we compared mutation counts generated from all sequences produced by TGCA bladder cancer whole exome sequencing with counts generated after first subsetting TCGA data to only those reads that coincide with the MSK-IMPACT capture regions. Because the data is not normally distributed, we use Spearman's correlation to examine the strength of association. The Cancer Genome Atlas (TCGA) dataset was accessed via cBioPortal (www.cbioportal.org/; last updated January 28, 2016) and FireHose (https://gdac.broadinstitute.org/; last updated January 28, 2016).
Statistical Methods
Patient and disease characteristics were compared using Fisher's exact test when categorical and Kruskal-Wallis test when continuous. Tests of trend across categories of number of DDR alterations were conducted. Best response was assessed based on RECIST v1.1 criteria. OS was calculated from the start date of platinum-based chemotherapy to the date of death of last follow-up. Patients still alive at last follow-up were censored for OS. PFS was calculated from the start date of platinum-based chemotherapy to the date of progression, death, or last follow-up. Patients alive and without progression were censored for PFS. For the TCGA cohort, OS and disease-free survival were calculated from the date of cystectomy. Cox regression was used to estimate associations between factors of interest and OS and PFS. Multivariable Cox regression included genetic factors of interest and adjusted for patient and disease characteristics determined based on a combination of clinical and statistical considerations. Additional exploratory analyses were performed based on potentially deleterious DDR alterations status, first with deleterious mutations as determined by in silico analysis and second with deleterious mutations as determined by literature review, as described above. Trend tests were conducted using logistic regression when the outcome was binary or linear regression with log-transformation for continuous outcomes.
A p-value <0.05 was considered statistically significant. All analyses were conducted using R software version 3.1.1 (R Core Development Team, Vienna, Austria).
Results
Patient Cohort
One hundred patients underwent pretreatment tumor sequencing and met the study entry critera between 10/2012 and 9/2015. The median age of the cohort was 66 years (range: 41 – 85) with the majority being males (67.0%), which is reflective of urothelial cancer patients. Primary sites were mostly bladder (n = 82), upper urinary tract (n=14) and urethra (4). Most patients (61.0%) had visceral metastases (hepatic, pulmonary or osseous), and 56.0% received a cisplatin-based regimen while the remaining received carboplatin-based treatment. Most received gemcitabine-based regimen (83.3%) while the remainder received carboplatin/paclitaxel (n=5), multi-drug regimens (ITP, GTP, MVAC, n=8) and two received carboplatin monotherapy (Table 1).
Table 1. Patient and disease characteristics by DDR alteration status.
| Overall | No DDR Alterations | Any DDR Alterations | p-value | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | % | n | % | n | % | |||
|
| ||||||||
| Age | Median | 66 | 67 | 65 | 0.226 | |||
| Range | 41 - 85 | 46 - 85 | 41 - 85 | |||||
|
| ||||||||
| Gender | Female | 23 | 23.0 | 18 | 34.6 | 5 | 10.4 | 0.052 |
| Male | 67 | 67.0 | 30 | 57.7 | 37 | 77.1 | ||
| N/A | 10 | 10.0 | 4 | 7.7 | 6 | 12.5 | ||
|
| ||||||||
| ECOG | 0 - 1 | 93 | 93.0 | 47 | 90.4 | 46 | 95.8 | 0.442 |
| ≥2 | 7 | 7.0 | 5 | 9.6 | 2 | 4.2 | ||
|
| ||||||||
| Visceral Disease (Hepatic, Pulmonary, Osseous) | Yes | 61 | 61.0 | 34 | 65.4 | 27 | 56.2 | 0.154 |
| No | 39 | 39.0 | 18 | 34.6 | 21 | 43.8 | ||
|
| ||||||||
| Primary Site | Bladder | 82 | 82.0 | 45 | 84.9 | 37 | 78.7 | 0.490 |
| Upper tract | 14 | 14.0 | 7 | 13.2 | 7 | 14.9 | ||
| Urethra | 4 | 4.0 | 1 | 1.9 | 3 | 6.4 | ||
|
| ||||||||
| Platinum type | Cisplatin | 56 | 56.0 | 24 | 45.3 | 32 | 68.1 | 0.027 |
| Carboplatin | 44 | 44.0 | 29 | 54.7 | 15 | 31.9 | ||
|
| ||||||||
| Prior Peri-operative Chemo | Yes | 14 | 13.7 | 10 | 18.9 | 4 | 8.5 | 0.082 |
| No | 77 | 86.3 | 34 | 64.2 | 43 | 91.5 | ||
| N/A | 9 | 9.0 | 9 | 17.0 | ||||
DDR Status and Baseline Characteristics
Forty-seven patients harbored alterations in DDR genes. Most of these patients had only 1 alteration (28/47; 59.6%), while 10 had 2 alterations and 9 had three or more alterations.
More patients were treated with cisplatin in the subgroup with DDR alterations (68.1% vs. 45.3%, p=0.027). Frequency of visceral metastases (53.2% vs. 67.9%, p=0.154) and other clinicopathologic characteristics were equally distributed (Table 1).
Clinical Outcomes
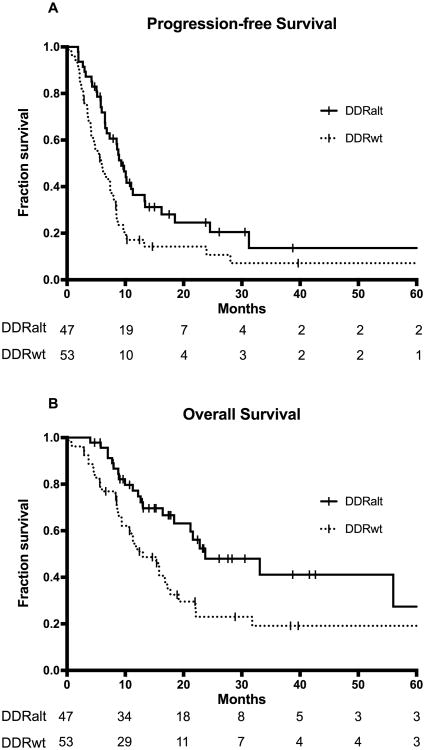
Median follow-up time among patients alive without progression was 14.1 months (range: 2.9 - 65.3). Median PFS for the entire cohort was 7.4 months (95% CI 6.2 – 8.8), which is similar to published reports.1,18 Patients with DDR alterations had significantly improved PFS of 9.3 months (95% CI: 7.3 – 13.4) compared to 6.0 months (95% CI 4.5 – 8.4) for those without DDR alterations (log rank p=0.007) (Figure 1A). DDR alterations, performance status, platinum type, and prior peri-operative platinum were significantly associated with PFS in uni- and multivariable analyses(Table 2).
Figure 1.
The association between DDR (DNA damage response and repair) gene alteration status and (A) progression-free (PFS) and (B) overall survival (OS). DDRalt: altered DDR; DDRwt: wild type DDR.
Table 2. Univariable and Multivariable Analyses for Progression-Free Survival and Overall Survival.
| Variables | Univariable Analysis | Multivariable Analysis | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | p | Hazard Ratio | p | Hazard Ratio | p | Hazard Ratio | p | |
| Age | 1.01 (0.99 - 1.04) | 0.356 | 1.01 (0.98 - 1.04) | 0.636 | ||||
|
| ||||||||
| Gender | ||||||||
| Female | 1.00 | 0.185 | 1.00 | 0.881 | ||||
| Male | 0.72 (0.44 - 1.17) | 0.96 (0.53 - 1.74) | ||||||
|
| ||||||||
| ECOG PS | ||||||||
| 0 - 1 | 1.00 | 0.013 | 1.00 | 0.040 | 1.00 | 0.023 | 1.00 | 0.012 |
| ≥2 | 2.71 (1.24 - 5.93) | 2.75 (1.05 - 7.20) | 2.69 (1.15 - 6.31) | 3.92 (1.36 - 11.36) | ||||
|
| ||||||||
| Visceral disease | ||||||||
| No | 1.00 | 0.290 | 1.00 | 0.552 | 1.00 | 0.014 | 1.00 | 0.077 |
| Yes | 1.29 (0.80 - 2.08) | 1.17 (0.70 - 1.95) | 2.08 (1.16 - 3.75) | 1.76 (0.94 - 3.29) | ||||
|
| ||||||||
| Platinum type | ||||||||
| Carboplatin | 1.00 | 0.008 | 1.00 | 0.025 | 1.00 | 0.016 | 1.00 | 0.354 |
| Cisplatin | 0.55 (0.35 - 0.86) | 0.54 (0.32 - 0.93) | 0.52 (0.31 - 0.88) | 0.75 (0.41 - 1.38) | ||||
|
| ||||||||
| Prior peri-operative chemotherapy | ||||||||
| No | 1.00 | 0.004 | 1.00 | 0.007 | 1.00 | 0.172 | 1.00 | 0.865 |
| Yes | 2.44 (1.32 - 4.48) | 2.72 (1.31 - 5.63) | 1.62 (0.81 - 3.23) | 1.08 (0.47 - 2.48) | ||||
|
| ||||||||
| DDR Alterations | ||||||||
| No | 1.00 | 0.007 | 1.00 | 0.046 | 1.00 | 0.008 | 1.00 | 0.029 |
| Yes | 0.54 (0.34 - 0.85) | 0.59 (0.35 - 0.99) | 0.48 (0.28 - 0.82) | 0.48 (0.25 - 0.93) | ||||
Abbreviations: DDR, DNA damage and response genes; PS, performance status; Visceral disease included hepatic, pulmonary and osseous metastases
Median follow-up time among survivors was 17.4 months (range: 2.9 – 104.5); median OS in the entire cohort was 17.8 months (95% CI 13.0 – 23.7). Patients with DDR alterations had significantly longer OS of 23.7 months (95% CI: 18.4 - not reached) compared to 13.0 months (95% CI: 10.7 – 19.2) for those without DDR alterations (log-rank p=0.006)(Figure 1B). In univariable analysis, performance status, visceral metastases and platinum type were significantly correlated with OS. In multivariable analysis, only performance status and DDR alteration remain independent prognostic indicators for OS. (Table 2) As more patients with DDR alteration were treated with cisplatin, we performed additional analysis to test for interaction between type of platinum chemotherapy and DDR status. No significant interaction was observed (p=0.938), suggesting that the effects of DDR alterations on OS are not different between patients who received carboplatin and those who received cisplatin.
Eighty-four patients were evaluable for objective response. Response rates were comparable regardless of DDR status (57.5% vs. 52.3%, p=0.666).
Predicted Impact of DDR Gene Alterations
Among the 47 patients with DDR alterations, 120 alterations were identified. Most were missense mutations (100/120, 83.3%) while the remaining were truncating (nonsense, splice site or frameshift alterations). Amongst the missense mutations, there were 95 unique mutations, of which only 13 were previously reported in COSMIC, published literature, or screening against a pan-cancer hotspot analysis of 11,119 tumors.17 In silico evaluation of these missense mutations identified 66% to be potentially deleterious according to either MutationAssessor or Polyphen-2. (Supplemental table 2)
When deleterious status was determined by in silico evaluation, 39% of the cohort was considered to harbor deleterious DDR alterations. There was no significant difference between those with deleterious and non-deleterious DDR alterations with regards to PFS (p=0.600) or OS (p=0.395). (Supplemental figures 1A & 1B).
When deleterious status was manually reviewed as per Iyer et al16, 28% of the cohort was considered to harbor deleterious DDR alterations. There was no significant difference between those with deleterious and non-deleterious DDR alterations with regards to PFS (p=0.724) or OS (p=0.640). (Supplemental figures 1C & 1D) When adjusted for other variables, those with deleterious DDR alterations demonstrated a trend towards improvement in PFS (HR 0.54, 95% CI 0.29 – 1.02, p=0.058) and statistically significant improvement in OS (HR 0.33, 95% CI 0.13 – 0.83, p=0.018) compared to those with wild type DDR genes. This trend towards improved PFS and OS was not observed with DDR alterations that were not considered deleterious (PFS: HR 0.66, 95% CI 0.34 – 1.28, p=0.216; OS: HR 0.66, 95% CI 0.30 – 1.47, p=0.311).
DDR Alteration Numbers and Mutational Burden
Although MSK-IMPACT contains a targeted panel of 341 genes and therefore covers only a fraction of the entire genome, the results constitute a strong surrogate for total mutation burden when applied to the TCGA dataset (Supplemental figure 2), with a Spearman correlation of r = 0.821, which represents strong correlation.
Compared to those without DDR alterations, patients with DDR alterations displayed a significantly higher number of mutations (Median 10 vs 7, p<0.001) and copy number alterations (median 3 vs 1, p<0.001)
Patients were divided into four subgroups based upon the number of DDR alterations (0, 1, 2, and ≥3). The number of DDR alterations was significantly associated with higher number of total mutations and copy number alterations (Supplemental figures 3A & 3B).
Increasing number of total mutations showed a trend towards increased response rate (odds ratio 1.06, 95% CI 0.99 – 1.14, p=0.091) and PFS (HR 0.97, 95% CI 0.98 – 1.00, p=0.079), while showing statistically significant OS improvement (HR 0.93, 95% CI 0.88 – 0.98, p=0.010). Adjusting for DDR status, trends towards improved response rates and OS were noted with increasing total mutation load (response rate: p=0.084, OS: p=0.056) but not for PFS (p=0.294). These relationships were graphically explored by examining mutation load as quartiles (Supplemental figures 4A-C).
DDR Alterations and Metastatic Disease Distribution
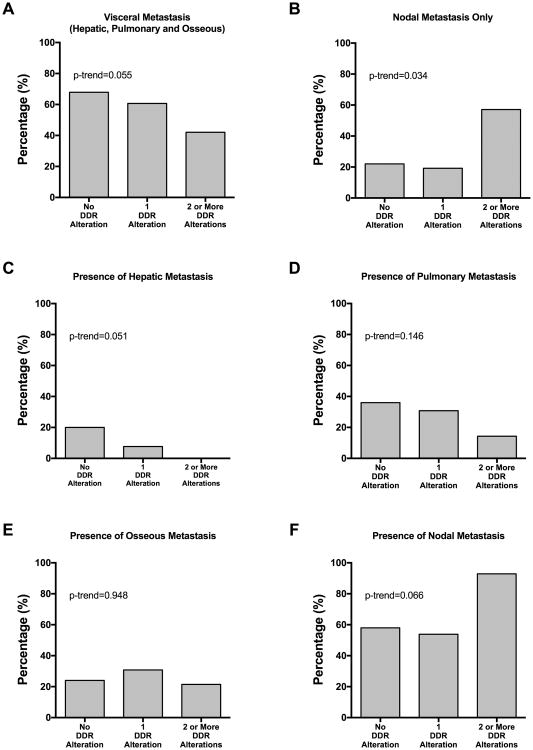
Clinical documentation of sufficient detail was available for 90 patients to explore the relationship between sites of metastatic involvement and DDR status. Visceral involvement showed an inverse trend with increasing number of DDR alterations (No DDR alteration/1 alteration/>1 alterations: 67.9%/60.7%/42.1%) (figure 2A), largely driven by low prevalence of hepatic (figure 2C) and pulmonary (figure 2D) involvement among DDR altered patients. (hepatic: 20.0%/7.7%/0%; pulmonary: 36.0%/30.8%/14.3%).
Figure 2.
Frequency of metastasis by site based on number of DDR alterations: (A) visceral metastasis, (B) nodal metastasis only (no visceral involvement), (C) any hepatic involvement, (D) any pulmonary involvement, (E) any osseous involvement and (F) any nodal involvement.
The opposite phenomenon was observed, with a positive correlation between increasing number of DDR alterations and node-only metastatic disease (figure 2B) (22.0%/19.2%/57.1%) or nodal metastasis regardless of other sites of disease (figure 2F) (58.0%/53.9%/92.9%).
Influence of Individual DNA Repair Pathways on Clinical Outcomes
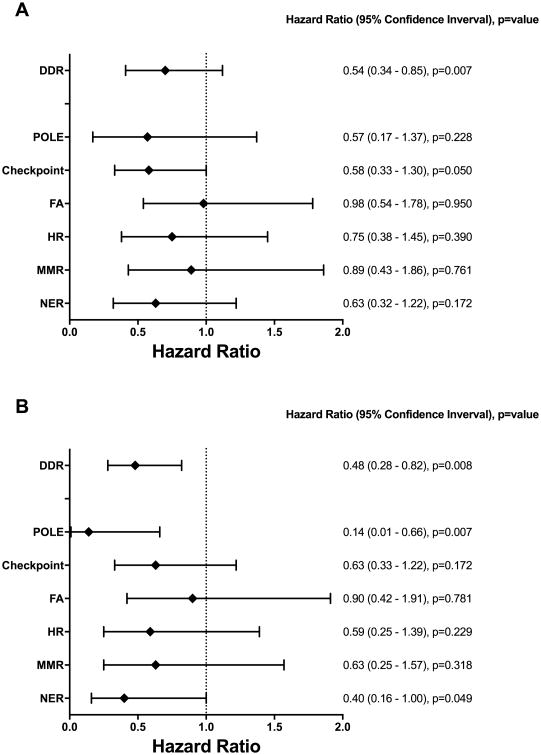
The DDR alterations were divided into alterations in individual DNA damage repair or response pathways: nucleotide excision repair (NER) (15%), mismatch repair (MMR) (9%), homologous recombination (HR) (11%), Fanconi anemia (FA) (16%), and DNA damage response checkpoint (23%). As POLE does not fall under any specific pathway and was altered in 6% of patients in the study, it was considered independently as well.
Alterations in different pathways exerted different magnitudes of effect on PFS and OS in exploratory analyses, most pronounced with POLE, damage response checkpoint and NER for PFS and POLE and NER for OS (Figures 3A, 3B).
Figure 3.
Influence of alterations in individual DDR pathways or components on (A) Progression-free survival and (B) overall survival.
Of the six cases with POLE alterations, two had significantly higher total number of alterations, at 27 and 42 alterations, respectively. The first of these had six different POLE alterations, including a nonsense mutation (R114*) and a missense mutation (P436H) within the exonuclease domain. The second harbored a missense alteration (R231H), also located within the exonuclease domain.
DDR Alterations and Clinical Outcomes in TCGA Cohort
The publicly available TCGA clinical and sequencing data were accessed, the most recent version which was updated on January, 28th 2016. However, only 112 patients have both complete clinical and sequencing data available on cBioPortal or Firehose. Of these patients, 74 patients were treated with only radical cystectomy while only 38 patients received adjuvant chemotherapy.
For those who were treated with radical cystectomy alone, DDR alteration status was not associated with both disease-free survival (hazard ratio 1.18, 95% CI 0.49 – 2.80, p=0.729) and OS (HR 0.77, 95% CI 0.42 – 1.42, p=0.408).
For the 38 patients who received radical cystectomy followed by adjuvant chemotherapy, DDR alteration status also was not associated with both disease-free survival (HR 0.43, 95% CI 0.12 – 1.48, p=0.181) and OS (HR 1.40, 95% CI 0.58 – 3.36, p=0.453).
Discussion
We examined the association between DDR alterations and clinical outcome in a cohort of urothelial carcinoma patients treated with first-line platinum-based chemotherapy. We observed that 48% of tumors contained alterations in DDR genes, and that their presence was an independent indicator of improved PFS and OS. Tumors with DDR gene alterations were also characterized by higher total mutation burden, lower rate of visceral metastases, and higher rate of nodal metastases.
Dysregulation of DNA repair genes has long been implicated in both carcinogenesis and prognosis of urothelial carcinoma. Polymorphisms in various DDR genes such as ERCC2 or NBN have previously been associated with the development of urothelial carcinoma19 and ERCC1 over-expression has been linked to poorer outcomes.20-23 The advent of next generation sequencing has allowed more in-depth interrogation of DDR pathways. In a study of 50 patients treated with cisplatin-based neoadjuvant chemotherapy, ERCC2 mutation was significantly enriched in the subset with ypT0/ypTis disease.11 Similarly, Plimack and colleagues demonstrated that alterations in one or more of the three DDR genes, ATM, RB1 and FANCC predicted increased likelihood of pathologic complete response following neoadjuvant cisplatin-based chemotherapy.10 In our study cohort, mutations in ERCC2, ATM and FANCC were only detected in 5, 9 and 1% of patients and, individually they were not significantly associated with clinical outcomes (data not shown). With a similar DDR gene panel detected on the MSK-IMPACT platform, we have also recently shown that presence of DDR alterations is associated with increased response to neoadjuvant chemotherapy16 and a trend towards improved outcomes to chemoradiation.24
Performing whole exome sequencing on 81 resected urothelial carcinoma cases with no prior neoadjuvant chemotherapy, Yap and colleagues observed an increased rate of mutations in ATM, ERCC2, FANCD2, PALB2, BRCA1 and BRCA2. Tumors with mutation in at least one of these genes had significantly higher overall numbers of somatic mutations25 and longer recurrence-free survival. Similarly, we observed that number of alterations in DDR genes was positively correlated with total number of mutations and copy number alterations. Although the MSK-IMPACT panel contained only 341 genes and therefore represents only a fraction of the entire genome, we demonstrated that the total mutation burden defined in our panel serves as an accurate surrogate for total mutation burden found using whole exome sequencing from the TCGA dataset, an approach that was also shown recently using the Foundation One assay in a large phase II clinical trial of atezolizumab26. Furthermore, our data also showed that tumors with higher mutation load also demonstrated a trend toward superior response and OS, independent of DDR status.
Recently, immune checkpoint blockade has revolutionized management of many disease types, including urothelial carcinoma. High mutation burden is associated with clinical benefit with checkpoint inhibitors26-29. Conversely, visceral metastatic involvement has been a recognized negative predictor of immunotherapy response 26 and overall survival 6. In this study, we observed an association between the number of DDR alterations, high mutation load, low visceral involvement and frequent nodal dissemination. Parallel phenomenon has been observed in microsatellite unstable colorectal cancer which is exquisitely sensitive to immune checkpoint inhibitors27. The underlying mechanistic etiology remains elusive, though it may bridge somatic DDR alterations, chemotherapy and immunotherapy in management of this lethal disease and is worthy of further investigation.
In our study cohort, alterations in POLE were observed in 6 patients. Three of these had only 1 DDR alteration and 3 had additional alterations, with a significantly higher number of total mutations compared to the rest of the cohort. In exploratory analyses, an improved trend towards PFS and significant improvement in OS were observed in POLE mutant patients. Mutations in POLE were also observed in 5% of the TCGA bladder cancer cohort. To date, POLE mutations have been associated with 1.5 – 2% of unselected colorectal cancer cases30,31 and 5.6 – 9.7% of endometrial carcinoma32,33. These tumors are characterized by an ultra-mutated phenotype and in high grade endometrial carcinoma, are associated with superior outcome32. Endometrial carcinomas with POLE mutations were found to contain significantly higher neoantigen load, a higher number of CD8+ tumor-infiltrating lymphocytes and over-expression of lymphocytic PD-1 as compared to tumors with microsatellite instability or wild-type tumors34. Although not confirmed in bladder cancer, these findings suggested that POLE mutations might represent a subset of clinically unique disease with better prognosis and potentially enhanced response to both chemotherapy and immunotherapy.
There are several limitations to our study. Due to the retrospective nature of this analysis, we cannot distinguish between DDR alteration's prognostic role and their function as predictors of platinum sensitivity or resistance. However, to investigate this, we evaluated whether DDR status is prognostic for survival in muscle invasive urothelial carcinoma not treated with perioperative chemotherapy based on available data from the bladder cohort of TCGA. Seventy-four patients received cystectomy alone, and DDR status did not appear to correlate with outcomes. No conclusions can be drawn regarding the association between DDR status and survival with adjuvant chemotherapy in the TCGA cohort, as only 38 patients with data available received adjuvant chemotherapy. Furthermore, since platinum-based chemotherapy is standard of care for metastatic urothelial carcinoma, it will require a prospective randomized trial involving a platinum-free chemotherapy arm to confirm the predictive role of DDR gene alterations. Secondly, our sample size is inadequate for more detailed investigation into the contribution of each DDR gene to the observed results. In addition, not all DDR genes are present in MSK-IMPACT. We attempted to overcome some of these limitations by grouping alterations within DDR pathways, each of which has shown variable degree of impact on clinical outcomes. Thirdly, not all alterations confer similar degree of functional impact on DNA damage response and repair capability, especially missense mutations which may result in varying hypomorphic effects. We used two different methodologies to identify potentially deleterious DDR alterations. Non-deleterious alterations as determined using in silico models were noted to demonstrate comparable OS and PFS advantages, as compared to those predicted to be deleterious. It is possible that some of these alterations were novel functional alterations that remain uncharacterized but are in fact deleterious. On the other hand, the more stringent selection criteria in our second approach16 appeared to show numerically superior OS, and thus could serve as a selection criteria for future validation efforts.
In conclusion, our study demonstrates that defects in DDR genes are prevalent in advanced urothelial carcinoma and have significant impact upon sensitivity to platinum therapy. This is consistent with our group's prior observation on DDR genes' influence on localized muscle-invasive urothelial carcinoma of the bladder treated with neoadjuvant cisplatin-based chemotherapy16 and chemoradiotherapy.24 The prognostic and predictive contributions of individual genes and pathways will be further investigated and validated in prospectively collected tumor specimens from a recently completed phase III trial of gemcitabine and cisplatin with or without bevacizumab (CALGB 90601). DDR gene alterations might also provide the missing link between platinum sensitivity and immunotherapy responsiveness via modulation of mutation burden, and further studies are required to further elucidate this relationship.
Supplementary Material
Translational Relevance.
Platinum-based chemotherapy remains the standard of care for advanced urothelial carcinoma. However, toxicity rate is high and only subset of patients benefits from therapy. There is no validated biomarker to date to guide treatment for these patients with lethal urothelial carcinoma.
Next generation sequencing efforts have characterized the genomic landscape of urothelial carcinoma. Utilizing our institutional CLIA-certified and NGS platform, this study identified a high rate of somatic alterations in DNA damage response and repair mechanisms which were associated with improved clinical outcomes.
We are currently validating our observation with tissues from the large phase III CALGB 90601trial. This can constitute the first step towards personalized treatment selection for patients with advanced urothelial carcinoma with a validated clinical sequencing platform, where only patients with defective DNA damage repair mechanism are offered platinum-based chemotherapy while clinical trials or immunotherapy can be considered for the other patients.
Acknowledgments
Funding: This work was supported by the Starr and Beene foundation grants and in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of interest disclosures: Jonathan E. Rosenberg has a patent issued for ERCC2 chemotherapy sensitivity. The remaining authors made no disclosures.
References
- 1.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 2.Bamias A, Dafni U, Karadimou A, et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03) Ann Oncol. 2013;24:1011–7. doi: 10.1093/annonc/mds583. [DOI] [PubMed] [Google Scholar]
- 3.De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30:191–9. doi: 10.1200/JCO.2011.37.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nogue-Aliguer M, Carles J, Arrivi A, et al. Gemcitabine and carboplatin in advanced transitional cell carcinoma of the urinary tract: an alternative therapy. Cancer. 2003;97:2180–6. doi: 10.1002/cncr.10990. [DOI] [PubMed] [Google Scholar]
- 5.Shannon C, Crombie C, Brooks A, Lau H, Drummond M, Gurney H. Carboplatin and gemcitabine in metastatic transitional cell carcinoma of the urothelium: effective treatment of patients with poor prognostic features. Ann Oncol. 2001;12:947–52. doi: 10.1023/a:1011186104428. [DOI] [PubMed] [Google Scholar]
- 6.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17:3173–81. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–65. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim PH, Cha EK, Sfakianos JP, et al. Genomic predictors of survival in patients with high-grade urothelial carcinoma of the bladder. Eur Urol. 2015;67:198–201. doi: 10.1016/j.eururo.2014.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder Cancer. Eur Urol. 2015;68:959–67. doi: 10.1016/j.eururo.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014;4:1140–53. doi: 10.1158/2159-8290.CD-14-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu D, Plimack ER, Hoffman-Censits J, et al. Clinical Validation of Chemotherapy Response Biomarker ERCC2 in Muscle-invasive Urothelial Bladder Carcinoma. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.1056. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17:251–64. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reva B, Antipin Y, Sander C. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res. 2011;39:e118. doi: 10.1093/nar/gkr407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer G, Balar AV, Milowsky MI, et al. Correlation of DNA damage response (DDR) gene alterations with response to neoadjuvant (neo) dose-dense gemcitabine and cisplatin (ddGC) in urothelial carcinoma (UC) ASCO Meeting Abstracts. 2016;34:5011. [Google Scholar]
- 17.Chang MT, Asthana S, Gao SP, et al. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat Biotechnol. 2016;34:155–63. doi: 10.1038/nbt.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol. 2012;30:1107–13. doi: 10.1200/JCO.2011.38.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stern MC, Lin J, Figueroa JD, et al. Polymorphisms in DNA repair genes, smoking, and bladder cancer risk: findings from the international consortium of bladder cancer. Cancer Res. 2009;69:6857–64. doi: 10.1158/0008-5472.CAN-09-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellmunt J, Paz-Ares L, Cuello M, et al. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Ann Oncol. 2007;18:522–8. doi: 10.1093/annonc/mdl435. [DOI] [PubMed] [Google Scholar]
- 21.Kim KH, Do IG, Kim HS, et al. Excision repair cross-complementation group 1 (ERCC1) expression in advanced urothelial carcinoma patients receiving cisplatin-based chemotherapy. APMIS. 2010;118:941–8. doi: 10.1111/j.1600-0463.2010.02648.x. [DOI] [PubMed] [Google Scholar]
- 22.Mullane SA, Werner L, Guancial EA, et al. Expression Levels of DNA Damage Repair Proteins Are Associated With Overall Survival in Platinum-Treated Advanced Urothelial Carcinoma. Clin Genitourin Cancer. 2015 doi: 10.1016/j.clgc.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozcan MF, Dizdar O, Dincer N, et al. Low ERCC1 expression is associated with prolonged survival in patients with bladder cancer receiving platinum-based neoadjuvant chemotherapy. Urol Oncol. 2013;31:1709–15. doi: 10.1016/j.urolonc.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Desai NB, Scott SN, Zabor EC, et al. Genomic characterization of response to chemoradiation in urothelial bladder cancer. Cancer. 2016 doi: 10.1002/cncr.30219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yap KL, Kiyotani K, Tamura K, et al. Whole-exome sequencing of muscle-invasive bladder cancer identifies recurrent mutations of UNC5C and prognostic importance of DNA repair gene mutations on survival. Clin Cancer Res. 2014;20:6605–17. doi: 10.1158/1078-0432.CCR-14-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016 doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–9. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spier I, Holzapfel S, Altmuller J, et al. Frequency and phenotypic spectrum of germline mutations in POLE and seven other polymerase genes in 266 patients with colorectal adenomas and carcinomas. Int J Cancer. 2015;137:320–31. doi: 10.1002/ijc.29396. [DOI] [PubMed] [Google Scholar]
- 31.Jesinghaus M, Pfarr N, Endris V, et al. Genotyping of Colorectal Cancer for Cancer Precision Medicine: Results from the IPH Center for Molecular Pathology. Genes Chromosomes Cancer. 2016 doi: 10.1002/gcc.22352. [DOI] [PubMed] [Google Scholar]
- 32.Billingsley CC, Cohn DE, Mutch DG, Hade EM, Goodfellow PJ. Prognostic Significance of POLE Exonuclease Domain Mutations in High-Grade Endometrioid Endometrial Cancer on Survival and Recurrence: A Subanalysis. Int J Gynecol Cancer. 2016 doi: 10.1097/IGC.0000000000000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Billingsley CC, Cohn DE, Mutch DG, Stephens JA, Suarez AA, Goodfellow PJ. Polymerase varepsilon (POLE) mutations in endometrial cancer: clinical outcomes and implications for Lynch syndrome testing. Cancer. 2015;121:386–94. doi: 10.1002/cncr.29046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howitt BE, Shukla SA, Sholl LM, et al. Association of Polymerase e-Mutated and Microsatellite-Instable Endometrial Cancers With Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015;1:1319–23. doi: 10.1001/jamaoncol.2015.2151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.