Abstract
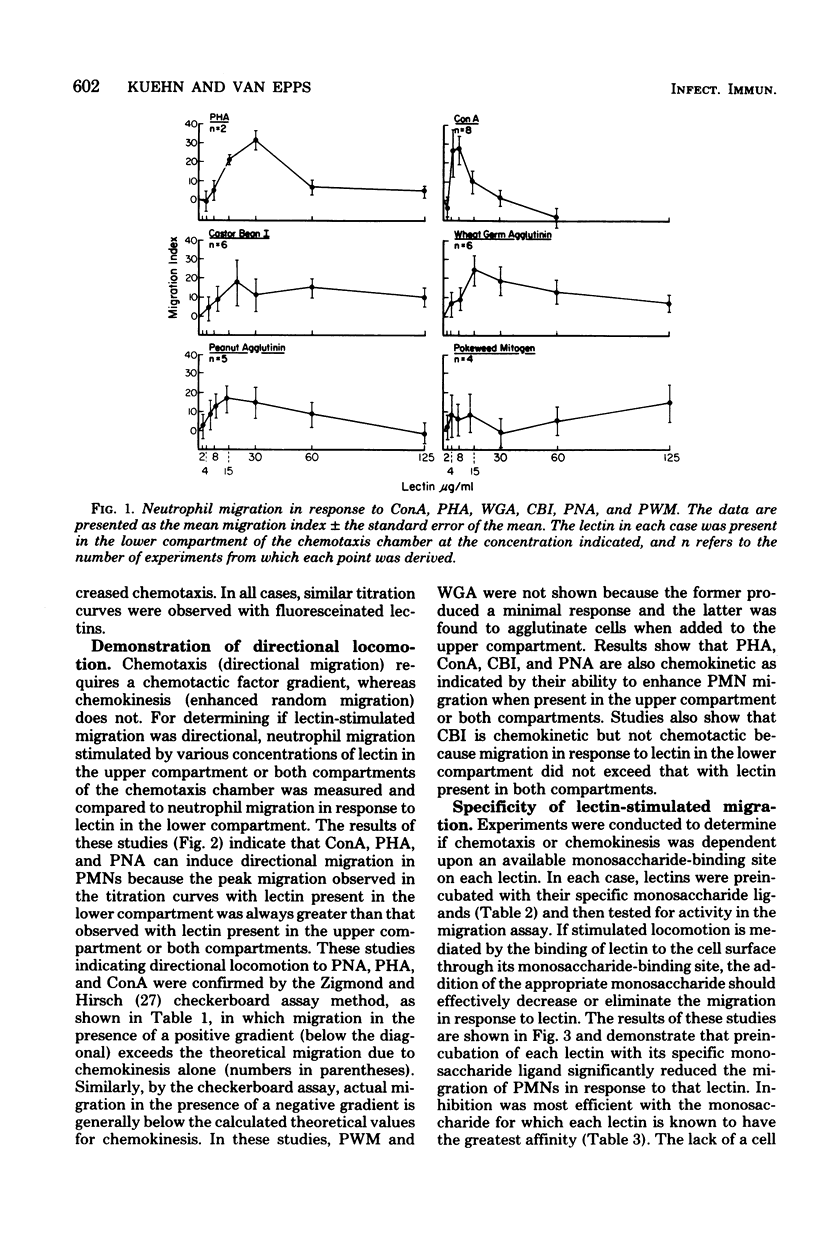
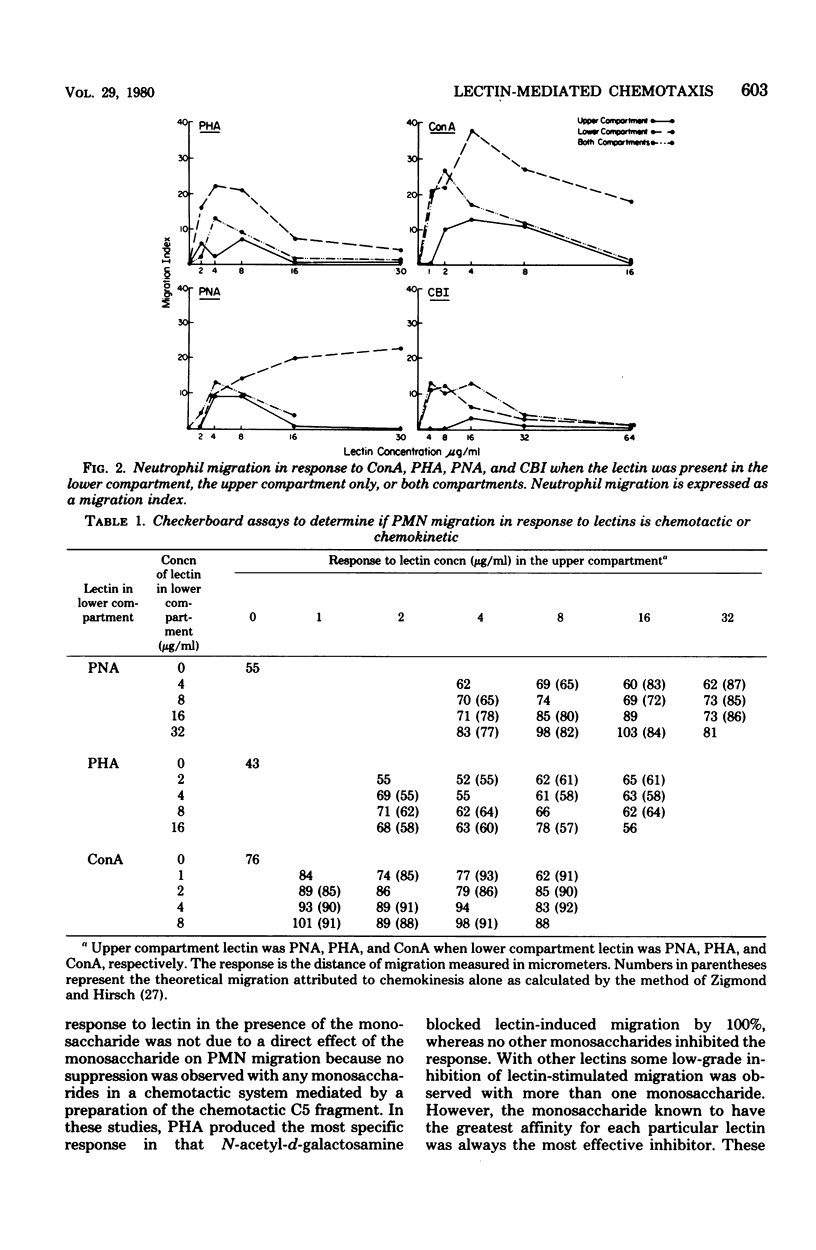
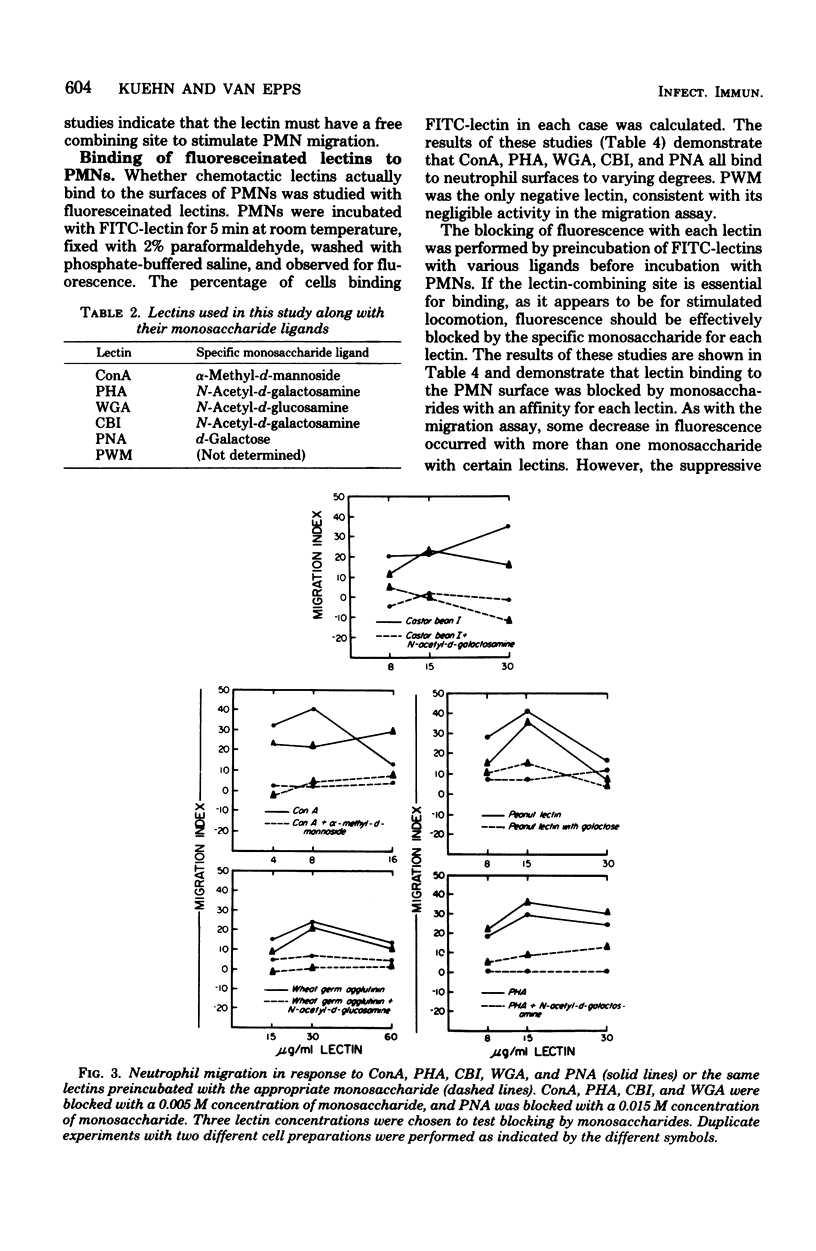
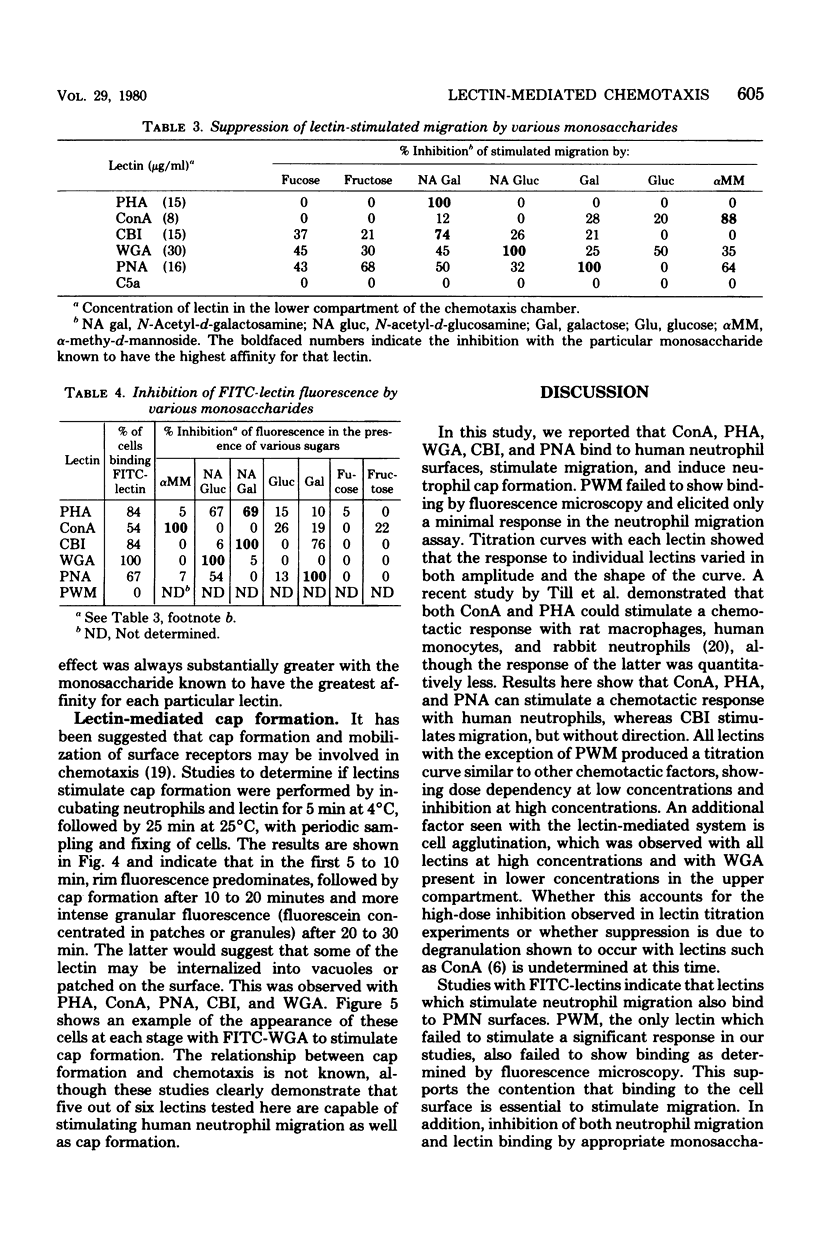
Six lectins, including concanavalin A, phytohemagglutinin P, castor bean I, wheat germ agglutinin, peanut agglutinin, and pokeweed mitogen, were studied for their ability to stimulate human neutrophil locomotion and cap formation. Five of these lectins with known monosaccharide specificities, including concanavalin A, phytohemagglutinin, P, castor bean I, wheat germ agglutinin, and peanut agglutinin, were found to stimulate human neutrophil migration in a modified Boyden assay. Pokeweed mitogen showed negligible activity in the locomotion assay as compared with other lectins. Tests were performed to determine if the observed neutrophil migration in response to lectins was directional, and it was found that concanavalin A, phytohemagglutinin P, and peanut agglutinin were both chemokinetic and chemotactic, whereas castor bean I was only chemokinetic. Wheat germ agglutinin could not be declared chemotactic or chemokinetic due to its tendency to agglutinate neutrophils. Studies with fluoresceinated lectins demonstrated that lectins which stimulate neutrophil migration also bind to neutrophil surfaces. Preincubation with specific monosaccharide ligands blocked both stimulated locomotion and fluorescence, suggesting that an available lectin-binding site was required both for lectin binding and the stimulation of migration. Additional experiments indicated that fluoresceinated concanavalin A, phytohemagglutinin P, castor bean I, wheat germ agglutinin, and peanut agglutinin all induce cap formation on the neutrophil.
Full text
PDF


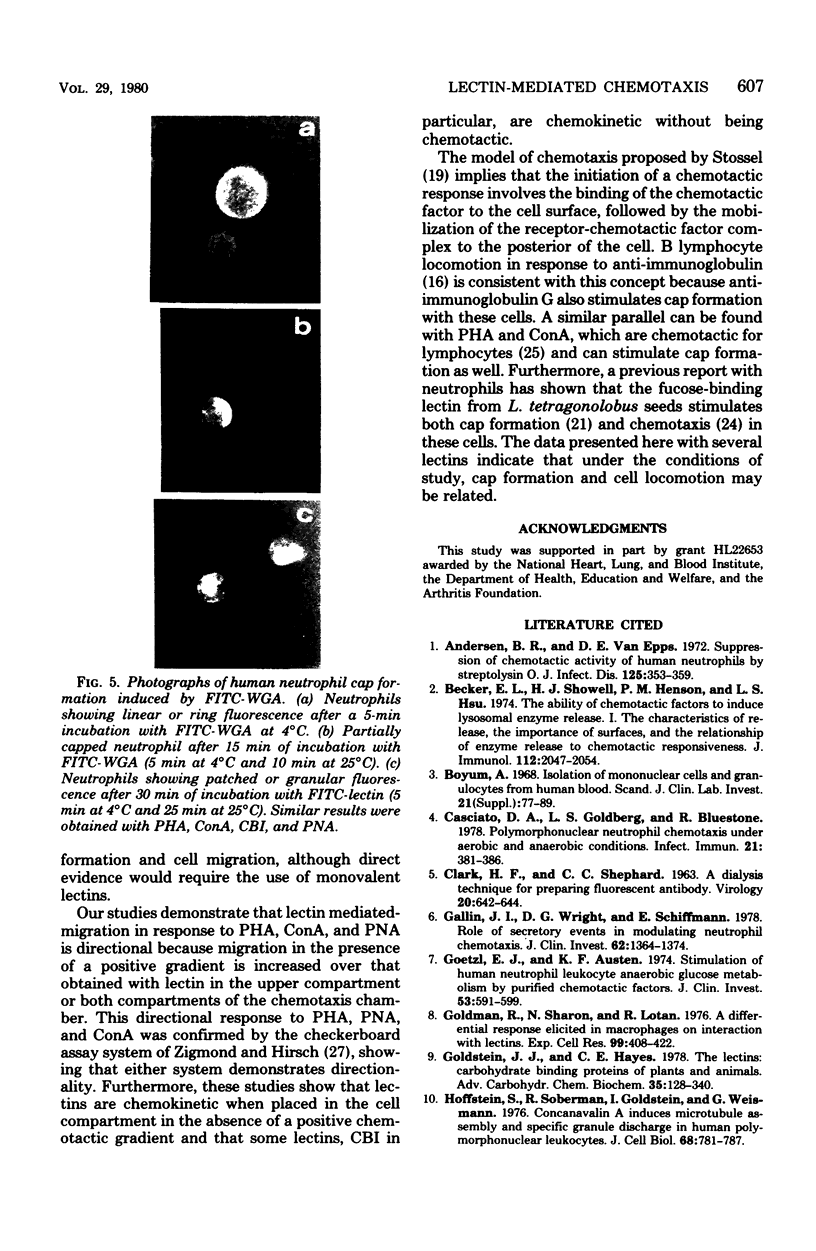

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B. R., Van Epps D. E. Suppression of chemotatic activity of human neutrophils by streptolysin O. J Infect Dis. 1972 Apr;125(4):353–359. doi: 10.1093/infdis/125.4.353. [DOI] [PubMed] [Google Scholar]
- Becker E. L., Showell H. J., Henson P. M., Hsu L. S. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol. 1974 Jun;112(6):2047–2054. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- CLARK H. F., SHEPARD C. C. A DIALYSIS TECHNIQUE FOR PREPARING FLUORESCENT ANTIBODY. Virology. 1963 Aug;20:642–644. doi: 10.1016/0042-6822(63)90292-7. [DOI] [PubMed] [Google Scholar]
- Casciato D. A., Goldberg L. S., Bluestone R. Polymorphonuclear neutrophil chemotaxis under aerobic and anaerobic conditions. Infect Immun. 1978 Aug;21(2):381–386. doi: 10.1128/iai.21.2.381-386.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Wright D. G., Schiffmann E. Role of secretory events in modulating human neutrophil chemotaxis. J Clin Invest. 1978 Dec;62(6):1364–1374. doi: 10.1172/JCI109257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. Stimulation of human neutrophil leukocyte aerobic glucose metabolism by purified chemotactic factors. J Clin Invest. 1974 Feb;53(2):591–599. doi: 10.1172/JCI107594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R., Sharon N., Lotan R. A differential response elicited in macrophages on interaction with lectins. Exp Cell Res. 1976 May;99(2):408–422. doi: 10.1016/0014-4827(76)90598-x. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Hoffstein S., Soberman R., Goldstein I., Weissmann G. Concanavalin A induces microtubule assembly and specific granule discharge in human polymorphonuclear leukocytes. J Cell Biol. 1976 Mar;68(3):781–787. doi: 10.1083/jcb.68.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 1975 Nov 10;250(21):8518–8523. [PubMed] [Google Scholar]
- Nagata Y., Burger M. M. Wheat germ agglutinin. Molecular characteristics and specificity for sugar binding. J Biol Chem. 1974 May 25;249(10):3116–3122. [PubMed] [Google Scholar]
- Nicolson G. L. The interactions of lectins with animal cell surfaces. Int Rev Cytol. 1974;39:89–190. doi: 10.1016/s0074-7696(08)60939-0. [DOI] [PubMed] [Google Scholar]
- Reisfeld R. A., Börjeson J., Chessin L. N., Small P. A., Jr Isolation and characterization of a mitogen from pokeweek (Phytolacca americana). Proc Natl Acad Sci U S A. 1967 Nov;58(5):2020–2027. doi: 10.1073/pnas.58.5.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G. B., Borysenko J. Z., Karnovsky M. J. Factors affecting the redistribution of surface-bound concanavalin A on human polymorphonuclear leukocytes. J Cell Biol. 1974 Aug;62(2):351–365. doi: 10.1083/jcb.62.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Anti-Ig-triggered movements of lymphocytes-specificity and lack of evidence for directional migration. J Immunol. 1975 Feb;114(2 Pt 2):809–814. [PubMed] [Google Scholar]
- Sharon N. Lectins. Sci Am. 1977 Jun;236(6):108-16, 118-9. doi: 10.1038/scientificamerican0677-108. [DOI] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till G., Lenhard V., Gemsa D. Chemotactic activity of lectins in vitro. Z Immunitatsforsch Immunobiol. 1978 Mar;154(2):173–185. [PubMed] [Google Scholar]
- Unanue E. R., Perkins W. D., Karnovsky M. J. Ligand-induced movement of lymphocyte membrane macromolecules. I. Analysis by immunofluorescence and ultrastructural radioautography. J Exp Med. 1972 Oct 1;136(4):885–906. doi: 10.1084/jem.136.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Bankhurst A. D., Williams R. C., Jr Casein-mediated neutrophil chemotaxis: a parallel between surface binding and chemotaxis. Inflammation. 1977 Jun;2(2):115–123. doi: 10.1007/BF00918673. [DOI] [PubMed] [Google Scholar]
- VanEpps D. E., Tung K. S. Fucose-binding Lotus tetragonolobus lectin binds to human polymorphonuclear leukocytes and induces a chemotactic response. J Immunol. 1977 Sep;119(3):1187–1189. [PubMed] [Google Scholar]
- Wilkinson P. C., Roberts J. A., Russell R. J., McLoughlin M. Chemotaxis of mitogen-activated human lymphocytes and the effects of membrane-active enzymes. Clin Exp Immunol. 1976 Aug;25(2):280–287. [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



