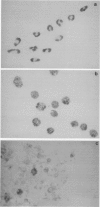
Abstract
Staphylococcal leukocidin is resolved by chromatography on carboxymethyl cellulose columns into two components, which are designated F (fast) and S (slow). Fixation and inactivation of both components were studied as follows. (i) Leukocidin activity was confined to the first 10 min of intoxication, and the maximal effect resulted from treating 106 rabbit peripheral polymorphonuclear leukocytes per 20 μl with 0.5 ng of each component of leukocidin. The S component was more responsible for the interaction with the leukocytes than the F component. (ii) The F component was inactivated by phosphatidylcholine at concentrations which corresponded to molar proportions of 1:1 and bound to [14C]phosphatidylcholine at equimolar proportions. (iii) The S component was inactivated by ganglioside GM1 at 1:1 molar proportions, but not by any of the related glycolipids. Ganglioside GM1 also was precipitated with the S component by a gel diffusion technique. Subunit B of cholera toxin competitively inhibited the binding of the S component to rabbit leukocyte membranes. This indicates that ganglioside GM1 may resemble or be part of the receptor site for the S component.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando S., Kon K., Isobe M., Yamakawa T. Structural study on tetraglycosyl ceremide and gangliosides isolated from human red blood cells. J Biochem. 1973 Apr;73(4):893–895. doi: 10.1093/oxfordjournals.jbchem.a130152. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., DAWSON R. M. The isolation of a new lipid, triphosphoinositide, and monophosphoinositide from ox brain. Biochem J. 1961 Dec;81:535–540. doi: 10.1042/bj0810535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLADSTONE G. P., VAN HEYNINGEN W. E. Staphylococcal leucocidins. Br J Exp Pathol. 1957 Apr;38(2):123–137. [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973 Aug;8(2):208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I., Naiki M. Ganglioside and rabbit erythrocyte membrane receptor for staphylococcal alpha-toxin. Infect Immun. 1976 Jan;13(1):289–291. doi: 10.1128/iai.13.1.289-291.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. T., Månsson J. E., Vanier M. T., Svennerholm L. Structure of the major glucosamine-containing ganglioside of human tissues. J Biol Chem. 1973 Apr 10;248(7):2634–2636. [PubMed] [Google Scholar]
- Naiki M., Marcus D. M., Ledeen R. Properties of antisera to ganglioside GM1 and asialo GM1. J Immunol. 1974 Jul;113(1):84–93. [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Luberoff D. E. A new method for separating lymphocytes and granulocytes from human peripheral blood using programmed gradient sedimentation in an isokinetic gradient. Immunology. 1973 Jan;24(1):85–92. [PMC free article] [PubMed] [Google Scholar]
- Siddiqui B., Hakomori S. A ceramide tetrasaccharide of human erythrocyte membrane reacting with anti-type XIV pneumococcal polysaccharide antiserum. Biochim Biophys Acta. 1973 Dec 13;330(2):147–155. doi: 10.1016/0005-2736(73)90219-8. [DOI] [PubMed] [Google Scholar]
- Soboll H., Ito A., Schaeg W., Blobel H. Leukozidin von Staphylokokken verschiedener Herkunft. Zentralbl Bakteriol Orig A. 1973 Jul;224(2):184–193. [PubMed] [Google Scholar]
- Van Heyningen W. E., Carpenter C. C., Pierce N. F., Greenough W. B., 3rd Deactivation of cholera toxin by ganglioside. J Infect Dis. 1971 Oct;124(4):415–418. doi: 10.1093/infdis/124.4.415. [DOI] [PubMed] [Google Scholar]
- WOODIN A. M. Fractionation of a leucocidin from Staphylococcus aureus. Biochem J. 1959 Oct;73:225–237. doi: 10.1042/bj0730225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODIN A. M. Purification of the two components of leucocidin from Staphylococcus aureus. Biochem J. 1960 Apr;75:158–165. doi: 10.1042/bj0750158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk K., Blobel H. Untersuchungen an "Leukozidinen" von Staphylokokken verschiedener Herkunft. Zentralbl Bakteriol Orig. 1970 May;213(4):479–487. [PubMed] [Google Scholar]
- Wiegandt H., Bücking H. W. Carbohydrate components of extraneuronal gangliosides from bovine and human spleen, and bovine kidney. Eur J Biochem. 1970 Aug;15(2):287–292. doi: 10.1111/j.1432-1033.1970.tb01006.x. [DOI] [PubMed] [Google Scholar]
- Woodin A. M., Wieneke A. A. Action of phospholipids and leucocidin on the p-nitrophenyl phosphatase of the leucocyte membrane. Biochim Biophys Acta. 1971 Jun 1;233(3):702–715. doi: 10.1016/0005-2736(71)90169-6. [DOI] [PubMed] [Google Scholar]
- Woodin A. M., Wieneke A. A. The action of phosphonates on the leucocyte in relation to the mode of action of leucocidin. The properties of the potassium pump and the inhibition of chemotaxis. Br J Exp Pathol. 1969 Jun;50(3):295–308. [PMC free article] [PubMed] [Google Scholar]
- Woodin A. M., Wieneke A. A. The interaction of eucocidin with the cell membrane of the polymorponuclearleucocyte. Biochem J. 1966 May;99(2):479–492. doi: 10.1042/bj0990479. [DOI] [PMC free article] [PubMed] [Google Scholar]