Abstract
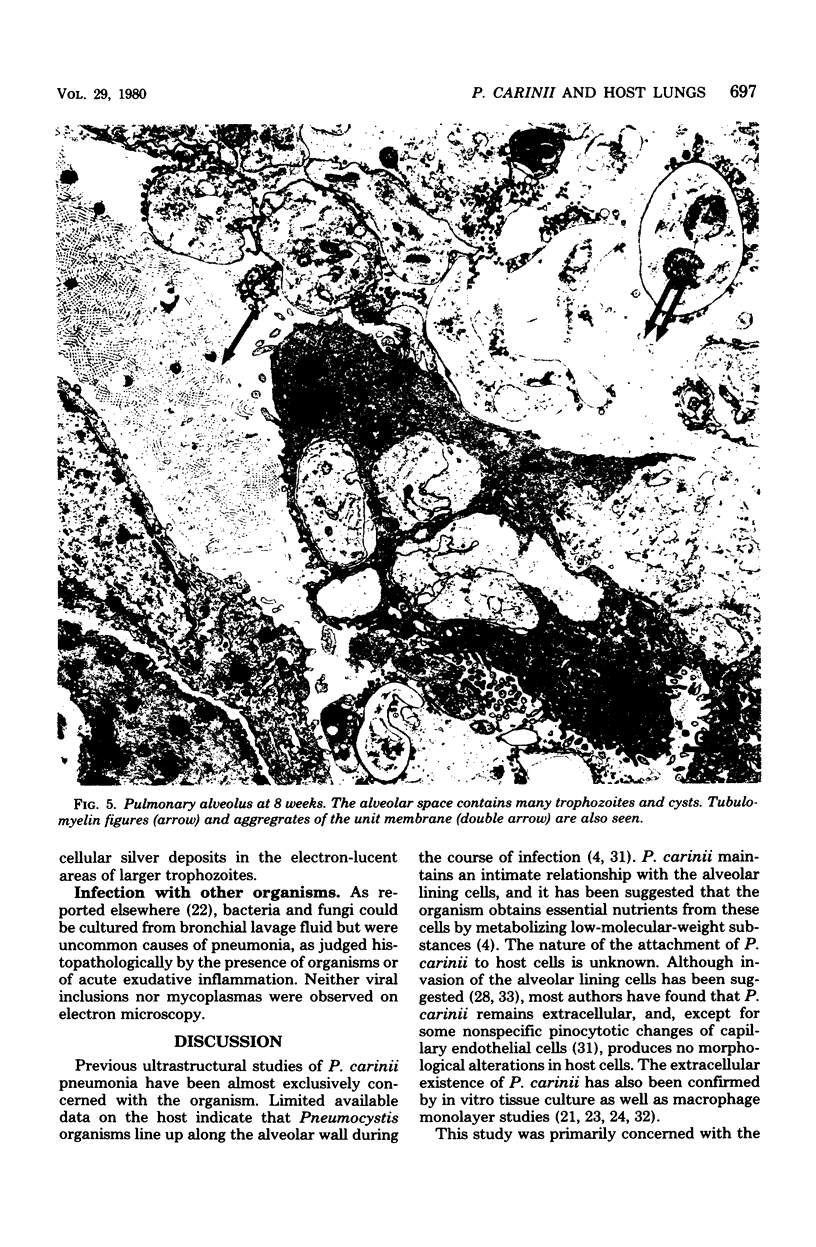
Pneumocystis carinii pneumonia was produced in rats by the administration of corticosteroids, low (8%) protein diet, and tetracycline in the drinking water. The rats were sacrificed at weekly intervals, and their lungs were examined by electron microscopy. For the first 6 weeks, few alterations were noted in host pulmonary tissue, except a close attachment of P. carinii trophozoites to the type I pneumocytes. At 7 to 8 weeks, when the infection reached the peak intensity on light microscopy, degenerative changes occurred in the type I pneumocyte, beginning with subepithelial bleb formation and followed by denudation of the basement membrane. This denuded surface appeared to be the site both of exudation of serum and tissue fluid into the alveolar space and of spread of P. carinii into the interstitium. There was hypertrophy of type II pneumocytes, which also occurred in uninfected control rats ingesting tetracyclines. With tapering of the corticosteroid dose, P. carinii was slowly cleared from the lungs, but latent infection persisted for at least 21 weeks. The host response to the corticosteroid dose tapering included increased prominence of alveolar macrophages and progressive interstitial lymphocytic infiltrate and fibrosis. Thus, P. carinii interacts with, and is associated with damage to, specific host cells. This interaction is important in the host-parasite relationship in this infection.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aso Y., Yoneda K., Kikkawa Y. Morphologic and biochemical study of pulmonary changes induced by bleomycin in mice. Lab Invest. 1976 Dec;35(6):558–568. [PubMed] [Google Scholar]
- Barton E. G., Jr, Campbell W. G., Jr Further observations on the ultrastructure of pneumocystis. Arch Pathol. 1967 Jun;83(6):527–534. [PubMed] [Google Scholar]
- Barton E. G., Jr, Campbell W. G., Jr Pneumocystis carinii in lungs of rats treated with cortisone acetate. Ultrastructural observations relating to the life cycle. Am J Pathol. 1969 Feb;54(2):209–236. [PMC free article] [PubMed] [Google Scholar]
- Brody A. R., Kelleher P. C., Craighead J. E. Mechanism of exudation through intact alveolar epithelial cells in the lungs of cytomegalovirus-infected mice. Lab Invest. 1978 Sep;39(3):281–288. [PubMed] [Google Scholar]
- Brooks R. E. Ruthenium red stainable surface layer on lung alveolar cells; electron microscopic interpretation. Stain Technol. 1969 Jul;44(4):173–177. doi: 10.3109/10520296909063346. [DOI] [PubMed] [Google Scholar]
- Campbell W. G., Jr Ultrastructure of Pneumocystis in human lung. Life cycle in human pneumocystosis. Arch Pathol. 1972 Apr;93(4):312–324. [PubMed] [Google Scholar]
- Coalson J. J., Beller J. J., Greenfield L. J. Effects of 100 per cent. oxygen ventilation on pulmonary ultrastructure and mechanics. J Pathol. 1971 Aug;104(4):267–273. doi: 10.1002/path.1711040409. [DOI] [PubMed] [Google Scholar]
- Dhir S. P., Boatman E. S. Location of polysaccharide on Chlamydia psittaci by silver-methenamine staining and electron microscopy. J Bacteriol. 1972 Jul;111(1):267–271. doi: 10.1128/jb.111.1.267-271.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Cabral L. J., Stephens R. J., Freeman G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am J Pathol. 1973 Feb;70(2):175–198. [PMC free article] [PubMed] [Google Scholar]
- Frenkel J. K., Good J. T., Shultz J. A. Latent Pneumocystis infection of rats, relapse, and chemotherapy. Lab Invest. 1966 Oct;15(10):1559–1577. [PubMed] [Google Scholar]
- Gil J., Weibel E. R. Improvements in demonstration of lining layer of lung alveoli by electron microscopy. Respir Physiol. 1969 Dec;8(1):13–36. doi: 10.1016/0034-5687(69)90042-5. [DOI] [PubMed] [Google Scholar]
- Gold J., L'Heureux P., Dehner L. P. Ultrastructure in the differential diagnosis of pulmonary histiocytosis and pneumocystosis. Arch Pathol Lab Med. 1977 May;101(5):243–247. [PubMed] [Google Scholar]
- Gottschall J. L., Walzer P. D., Yoneda K. The morphologic changes of the rat type II pneumocytes induced by oxytetracycline. Lab Invest. 1979 Jul;41(1):5–12. [PubMed] [Google Scholar]
- Ham E. K., Greenberg D., Reynolds R. C., Singer D. B. Ultrastructure of Pneumocystis carinii. Exp Mol Pathol. 1971 Jun;14(3):362–372. doi: 10.1016/0014-4800(71)90007-4. [DOI] [PubMed] [Google Scholar]
- Hughes W. T. Limited effect of trimethoprim-sulfamethoxazole prophylaxis on Pneumocystis carinii. Antimicrob Agents Chemother. 1979 Sep;16(3):333–335. doi: 10.1128/aac.16.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W. T., Price R. A., Sisko F., Havron W. S., Kafatos A. G., Schonland M., Smythe P. M. Protein-calorie malnutrition. A host determinant for Pneumocystis carinii infection. Am J Dis Child. 1974 Jul;128(1):44–52. doi: 10.1001/archpedi.1974.02110260046008. [DOI] [PubMed] [Google Scholar]
- Masur H., Jones T. C. The interaction in vitro of Pneumocystis carinii with macrophages and L-cells. J Exp Med. 1978 Jan 1;147(1):157–170. doi: 10.1084/jem.147.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. J., Pifer L. L., Hughes W. T. Pneumocystis carinii in vitro: A study by scanning electron microscopy. Am J Pathol. 1977 Feb;86(2):387–401. [PMC free article] [PubMed] [Google Scholar]
- Pine J. H., Richter W. R., Esterly J. R. Experimental lung injury. I. Bacterial pneumonia: ultrastructural, autoradiographic and histochemical observations. Am J Pathol. 1973 Oct;73(1):115–130. [PMC free article] [PubMed] [Google Scholar]
- Price R. A., Hughes W. T. Histopathology of Pneumocystis carinii infestation and infection in malignant disease in childhood. Hum Pathol. 1974 Nov;5(6):737–752. doi: 10.1016/s0046-8177(74)80043-2. [DOI] [PubMed] [Google Scholar]
- Rapp F., Buss E. R. Are viruses important in carcinogenesis? Am J Pathol. 1974 Oct;77(1):85–102. [PMC free article] [PubMed] [Google Scholar]
- SHELDON W. H. Experimental pulmonary Pneumocystis carinii infection in rabbits. J Exp Med. 1959 Jul 1;110(1):147–160. doi: 10.1084/jem.110.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. N., Moe K. K., Dellers R. W. Fine structure of spontaneous Pneumocystis carinii pulmonary infection in foals. Cornell Vet. 1974 Jan;64(1):72–88. [PubMed] [Google Scholar]
- Sueishi K., Hisano S., Sumiyoshi A., Tanaka K. Scanning and transmission electron microscopic study of human pulmonary pneumocystosis. Chest. 1977 Aug;72(2):213–216. doi: 10.1378/chest.72.2.213. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Phillips B. P. Electron microscope studies of experimental Entamoeba histolytica infection in the guinea pig. I. Penetration of the intestinal epithelium by trophozoites. Am J Trop Med Hyg. 1975 Jan;24(1):34–48. doi: 10.4269/ajtmh.1975.24.34. [DOI] [PubMed] [Google Scholar]
- Vavra J., Kucera K. Pneumocystis carinii delanoë, its ultrastructure and ultrastructural affinities. J Protozool. 1970 Aug;17(3):463–483. doi: 10.1111/j.1550-7408.1970.tb04715.x. [DOI] [PubMed] [Google Scholar]
- Von Behren L. A., Pesanti E. L. Uptake and degradation of Pneumocystis carinii by macrophages in vitro. Am Rev Respir Dis. 1978 Dec;118(6):1051–1059. doi: 10.1164/arrd.1978.118.6.1051. [DOI] [PubMed] [Google Scholar]
- Vossen M. E., Beckers P. J., Meuwissen J. H., Stadhouders A. M. Developmental biology of Pneumocystis carinii, and alternative view on the life cycle of the parasite. Z Parasitenkd. 1978 Apr 20;55(2):101–118. doi: 10.1007/BF00384826. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Perl D. P., Krogstad D. J., Rawson P. G., Schultz M. G. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974 Jan;80(1):83–93. doi: 10.7326/0003-4819-80-1-83. [DOI] [PubMed] [Google Scholar]
- Walzer P. D. Pneumocystis carinii infection. South Med J. 1977 Nov;70(11):1330–1337. doi: 10.1097/00007611-197711000-00027. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Powell R. D., Jr, Yoneda K. Experimental Pneumocystis carinii pneumonia in different strains of cortisonized mice. Infect Immun. 1979 Jun;24(3):939–947. doi: 10.1128/iai.24.3.939-947.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Powell R. D., Jr, Yoneda K., Rutledge M. E., Milder J. E. Growth characteristics and pathogenesis of experimental Pneumocystis carinii pneumonia. Infect Immun. 1980 Mar;27(3):928–937. doi: 10.1128/iai.27.3.928-937.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Rutledge M. E., Yoneda K., Stahr B. J. Pneumocystis carinii: new separation method from lung tissue. Exp Parasitol. 1979 Jun;47(3):356–368. doi: 10.1016/0014-4894(79)90088-2. [DOI] [PubMed] [Google Scholar]
- Wang N. S., Huang S. N., Sheldon H., Thurlbeck W. M. Ultrastructural changes of Clara and type II alveolar cells in adrenalin-induced pulmonary edema in mice. Am J Pathol. 1971 Feb;62(2):237–252. [PMC free article] [PubMed] [Google Scholar]
- Wang N. S., Huang S. N., Thurlbeck W. M. Combined Pneumocystis carinii and cytomegalovirus infection. Arch Pathol. 1970 Dec;90(6):529–535. [PubMed] [Google Scholar]