Abstract
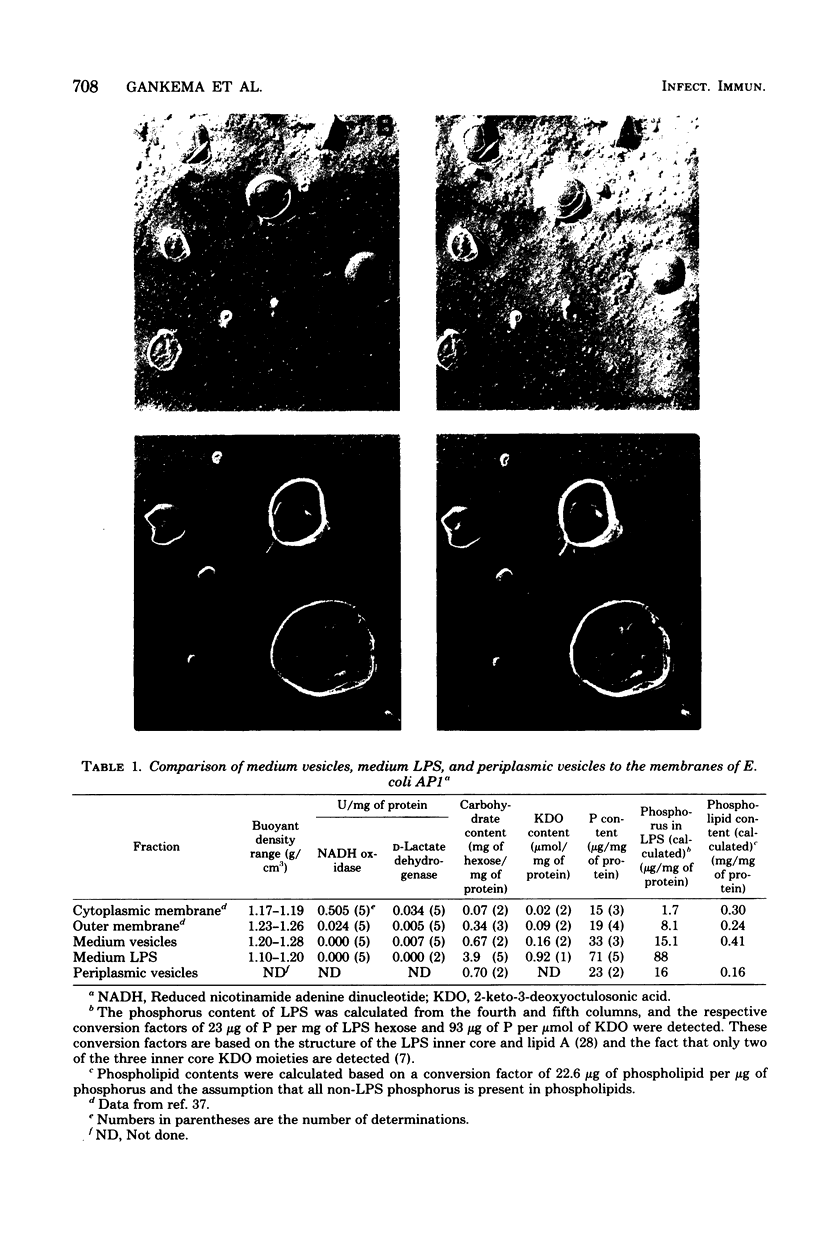
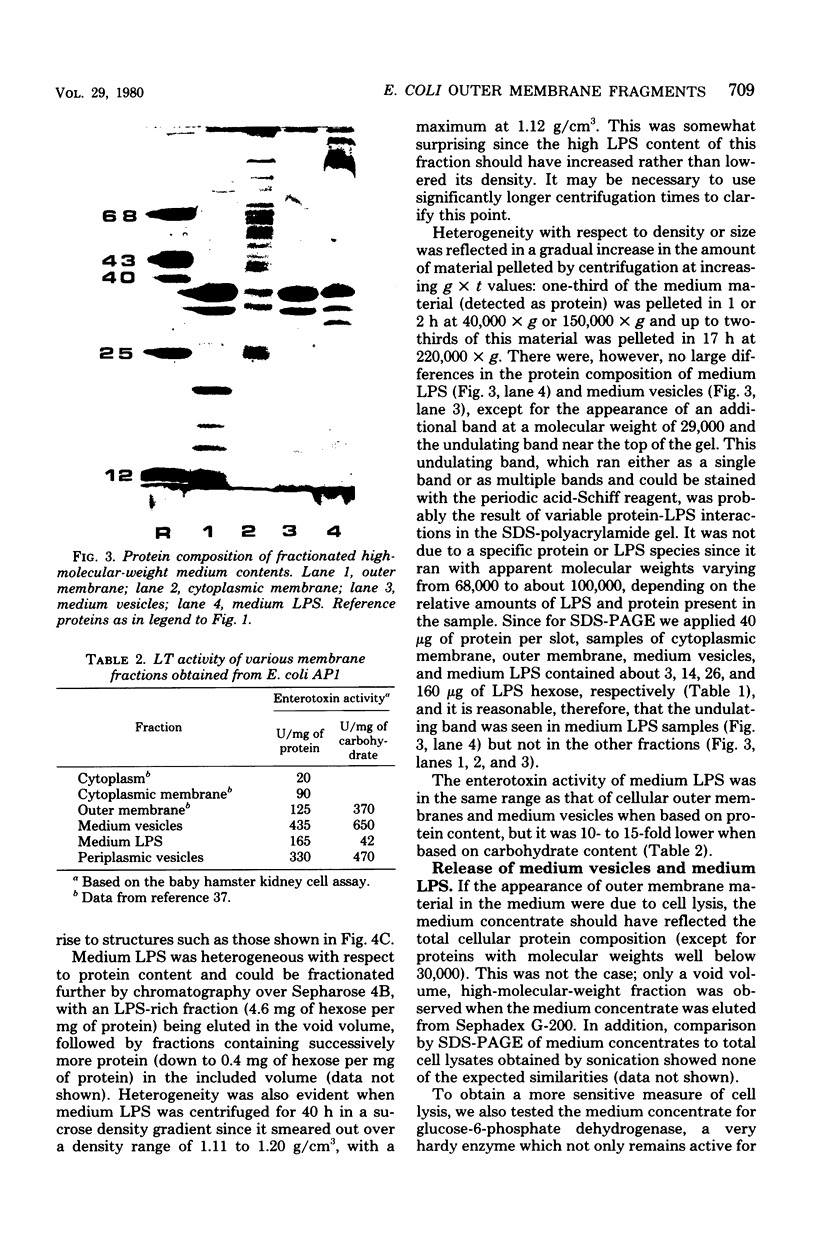
The high-molecular-weight material released into the medium by Escherichia coli AP1, an enterotoxigenic strain of porcine origin, has been isolated and resolved into two clearly distinct fractions, based on sucrose density gradient and differential centrifugation, chemical analysis, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and freeze-fracture electron microscopy. These two fractions, referred to as “medium vesicles” and “medium lipopolysaccharides”, were compared with the cellular outer and cytoplasmic membranes, the periplasmic fraction, and the cytoplasmic fraction. The medium vesicles closely resembled outer membrane and accounted for 3 to 5% of the total cellular outer membrane. They contained most of the heat-labile enterotoxin (LT) activity released into the medium by E. coli AP1. The medium lipopolysaccharide consisted mostly of lipopolysaccharide and a small amount of outer membrane and contained relatively little LT activity. Based on experiments with E. coli K-12 strains, in which about 5% of the newly synthesized outer membrane is lost from areas of outer membrane synthesis, it is proposed that enterotoxigenic E. coli strains release LT as part of such newly synthesized outer membrane fragments and that released outer membrane fragments may function as physiologically significant LT carriers.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clements J. D., Finkelstein R. A. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979 Jun;24(3):760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Dröge W., Lehmann V., Lüderitz O., Westphal O. Structural investigations on the 2-keto-3-deoxyoctonate region of lipopolysaccharides. Eur J Biochem. 1970 May 1;14(1):175–184. doi: 10.1111/j.1432-1033.1970.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Welsh K. M., Bayer M. E. Characterization of membrane-bound nicotinamide adenine dinucleotide glycohydrolase of Vibrio cholerae. J Biol Chem. 1979 Sep 25;254(18):9254–9261. [PubMed] [Google Scholar]
- Germanier R., Fürer E., Varallyay S., Inderbitzin T. M. Preparation of a purified antigenic cholera toxoid. Infect Immun. 1976 Jun;13(6):1692–1698. doi: 10.1128/iai.13.6.1692-1698.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., King C. A. The mechanism of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975 Aug 25;250(16):6424–6432. [PubMed] [Google Scholar]
- Hoekstra D., van der Laan J. W., de Leij L., Witholt B. Release of outer membrane fragments from normally growing Escherichia coli. Biochim Biophys Acta. 1976 Dec 14;455(3):889–899. doi: 10.1016/0005-2736(76)90058-4. [DOI] [PubMed] [Google Scholar]
- Jacks T. M., Wu B. J., Braemer A. C., Bidlack D. E. Properties of the enterotoxic component in Escherichia coli enteropathogenic for swine. Infect Immun. 1973 Feb;7(2):178–189. doi: 10.1128/iai.7.2.178-189.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Cullen J., Work E. An extracellular lipopolysaccharide-phospholipid-protein complex produced by Escherichia coli grown under lysine-limiting conditions. Biochem J. 1967 Apr;103(1):192–201. doi: 10.1042/bj1030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Vesk M., Work E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1206–1217. doi: 10.1128/jb.92.4.1206-1217.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel S. L., Robertson D. C. Purification and chemical characterization of the heat-labile enterotoxin produced by enterotoxigenic Escherichia coli. Infect Immun. 1979 Aug;25(2):586–596. doi: 10.1128/iai.25.2.586-596.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lariviére S., Gyles C. L., Barnum D. A. Preliminary characterization of the heat-labile enterotoxin of Escherichia coli F11(P155). J Infect Dis. 1973 Sep;128(3):312–320. doi: 10.1093/infdis/128.3.312. [DOI] [PubMed] [Google Scholar]
- Lehmann V., Rupprecht E. Microheterogeneity in lipid A demonstrated by a new intermediate in the biosynthesis of 3-deozy-D-manno-octulosonic-acid--lipid A. Eur J Biochem. 1977 Dec;81(3):443–452. doi: 10.1111/j.1432-1033.1977.tb11969.x. [DOI] [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Loeb M. R. Bacteriophage T4-mediated release of envelope components from Escherichia coli. J Virol. 1974 Mar;13(3):631–641. doi: 10.1128/jvi.13.3.631-641.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mug-Opstelten D., Witholt B. Preferential release of new outer membrane fragments by exponentially growing Escherichia coli. Biochim Biophys Acta. 1978 Apr 4;508(2):287–295. doi: 10.1016/0005-2736(78)90331-0. [DOI] [PubMed] [Google Scholar]
- Nagy B., Moon H. W., Isaacson R. E. Colonization of porcine small intestine by Escherichia coli: ileal colonization and adhesion by pig enteropathogens that lack K88 antigen and by some acapsular mutants. Infect Immun. 1976 Apr;13(4):1214–1220. doi: 10.1128/iai.13.4.1214-1220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Thompson R. C., Davis B. D. Extracellular labeling of nascent polypeptides traversing the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2830–2834. doi: 10.1073/pnas.74.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speirs J. I., Stavric S., Konowalchuk J. Assay of Escherichia coli heat-labile enterotoxin with vero cells. Infect Immun. 1977 May;16(2):617–622. doi: 10.1128/iai.16.2.617-622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnett J. D., Gilleland H. E., Jr, Eagon R. G. Proteins released from cell envelopes of Pseudomonas aeruginosa on exposure to ethylenediaminetetraacetate: comparison with dimethylformamide-extractable proteins. J Bacteriol. 1973 Apr;114(1):399–407. doi: 10.1128/jb.114.1.399-407.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckesser J., Drews G., Ladwig R. Localization and biological and physicochemical properties of the cell wall lipopolysaccharide of Rhodopseudomonas capsulata. J Bacteriol. 1972 Apr;110(1):346–353. doi: 10.1128/jb.110.1.346-353.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink J., Gankema H., Jansen W. H., Guinée P. A., Witholt B. Isolation of the membranes of an enterotoxigenic strain of Escherichia coli and distribution of enterotoxin activity in different subcellular fractions. Biochim Biophys Acta. 1978 Dec 4;514(1):128–136. doi: 10.1016/0005-2736(78)90082-2. [DOI] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- de Leij L., Kingma J., Witholt B. Insertion of newly synthesized proteins into the outer membrane of Escherichia coli. Biochim Biophys Acta. 1978 Sep 22;512(2):365–376. doi: 10.1016/0005-2736(78)90260-2. [DOI] [PubMed] [Google Scholar]
- de Leij L., Kingma J., Witholt B. Nature of the regions involved in the insertion of newly synthesized protein into the outer membrane of Escherichia coli. Biochim Biophys Acta. 1979 May 17;553(2):224–234. doi: 10.1016/0005-2736(79)90227-x. [DOI] [PubMed] [Google Scholar]
- de Smet M. J., Kingma J., Witholt B. The effect of toluene on the structure and permeability of the outer and cytoplasmic membranes of Escherichia coli. Biochim Biophys Acta. 1978 Jan 4;506(1):64–80. doi: 10.1016/0005-2736(78)90435-2. [DOI] [PubMed] [Google Scholar]
- van Heerikhuizen H., Kwak E., van Bruggen E. F., Witholt B. Characterization of a low density cytoplasmic membrane subfraction isolated from Escherichia coli. Biochim Biophys Acta. 1975 Dec 1;413(2):177–191. doi: 10.1016/0005-2736(75)90102-9. [DOI] [PubMed] [Google Scholar]