Abstract
Previous studies have shown that spermatogenic cells are a major source of testicular RNA encoding the opioid peptide precursor proenkephalin, suggesting that proenkephalin-derived peptides may function as intratesticular paracrine factors produced by male germ cells. However, direct evidence for the production of proenkephalin by spermatogenic cells has been lacking. In this report, we have used polysome profile analysis, peptide quantitation, and immunocytochemistry to show that proenkephalin products are synthesized during spermatogenesis and are retained within spermatozoa of humans, hamsters, rats, and sheep. We further show that these peptides are stored in the sperm acrosome and are depleted from sperm following the acrosome reaction, an exocytotic event required for fertilization. Proenkephalin products thus may serve a dual function as sperm acrosomal factors released during the fertilization process as well as intratesticular regulators secreted by spermatogenic cells.
Full text
PDF

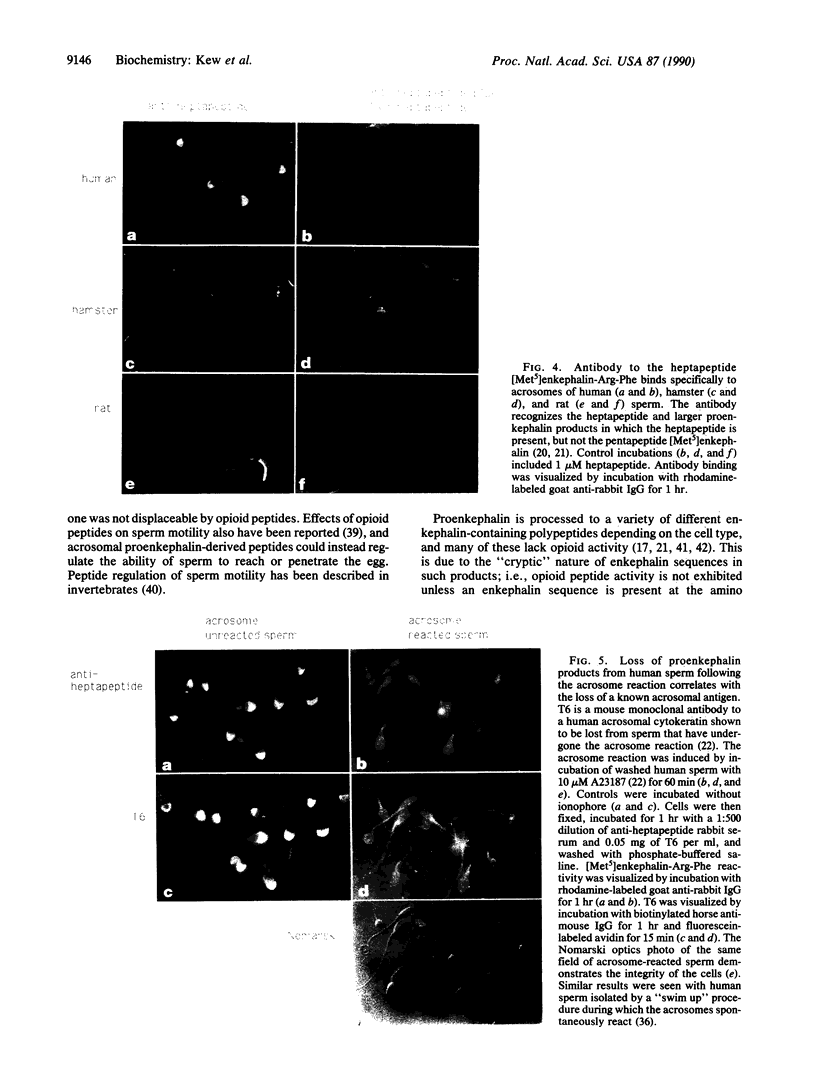

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakalkin GYa, Yakovleva T. V., Nikitina L. A., Korobov N. V., Vinogradov V. A., Titov M. I. Opiate binding sites and endogenous opioids in Bufo viridis oocytes. Biochem Biophys Res Commun. 1983 Dec 28;117(3):718–724. doi: 10.1016/0006-291x(83)91656-x. [DOI] [PubMed] [Google Scholar]
- Bellvé A. R., Millette C. F., Bhatnagar Y. M., O'Brien D. A. Dissociation of the mouse testis and characterization of isolated spermatogenic cells. J Histochem Cytochem. 1977 Jul;25(7):480–494. doi: 10.1177/25.7.893996. [DOI] [PubMed] [Google Scholar]
- Douglass J., Cox B., Quinn B., Civelli O., Herbert E. Expression of the prodynorphin gene in male and female mammalian reproductive tissues. Endocrinology. 1987 Feb;120(2):707–713. doi: 10.1210/endo-120-2-707. [DOI] [PubMed] [Google Scholar]
- Fabbri A., Knox G., Buczko E., Dufau M. L. Beta-endorphin production by the fetal Leydig cell: regulation and implications for paracrine control of Sertoli cell function. Endocrinology. 1988 Feb;122(2):749–755. doi: 10.1210/endo-122-2-749. [DOI] [PubMed] [Google Scholar]
- Fabbri A., Knox G., Buczko E., Dufau M. L. Beta-endorphin production by the fetal Leydig cell: regulation and implications for paracrine control of Sertoli cell function. Endocrinology. 1988 Feb;122(2):749–755. doi: 10.1210/endo-122-2-749. [DOI] [PubMed] [Google Scholar]
- Fleminger G., Lahm H. W., Udenfriend S. Changes in rat adrenal catecholamines and proenkephalin metabolism after denervation. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3587–3590. doi: 10.1073/pnas.81.11.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraioli F., Fabbri A., Gnessi L., Silvestroni L., Moretti C., Redi F., Isidori A. Beta-endorphin, Met-enkephalin, and calcitonin in human semen: evidence for a possible role in human sperm motility. Ann N Y Acad Sci. 1984;438:365–370. doi: 10.1111/j.1749-6632.1984.tb38297.x. [DOI] [PubMed] [Google Scholar]
- Garbers D. L. Molecular basis of fertilization. Annu Rev Biochem. 1989;58:719–742. doi: 10.1146/annurev.bi.58.070189.003443. [DOI] [PubMed] [Google Scholar]
- Garrett J. E., Collard M. W., Douglass J. O. Translational control of germ cell-expressed mRNA imposed by alternative splicing: opioid peptide gene expression in rat testis. Mol Cell Biol. 1989 Oct;9(10):4381–4389. doi: 10.1128/mcb.9.10.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J. E., Douglass J. O. Human chorionic gonadotropin regulates expression of the proenkephalin gene in adult rat Leydig cells. Mol Endocrinol. 1989 Dec;3(12):2093–2100. doi: 10.1210/mend-3-12-2093. [DOI] [PubMed] [Google Scholar]
- Gerendai I., Shaha C., Gunsalus G. L., Bardin C. W. The effects of opioid receptor antagonists suggest that testicular opiates regulate Sertoli and Leydig cell function in the neonatal rat. Endocrinology. 1986 May;118(5):2039–2044. doi: 10.1210/endo-118-5-2039. [DOI] [PubMed] [Google Scholar]
- Howells R. D., Kilpatrick D. L., Bailey L. C., Noe M., Udenfriend S. Proenkephalin mRNA in rat heart. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1960–1963. doi: 10.1073/pnas.83.6.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells R. D., Kilpatrick D. L., Bhatt R., Monahan J. J., Poonian M., Udenfriend S. Molecular cloning and sequence determination of rat preproenkephalin cDNA: sensitive probe for studying transcriptional changes in rat tissues. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7651–7655. doi: 10.1073/pnas.81.23.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
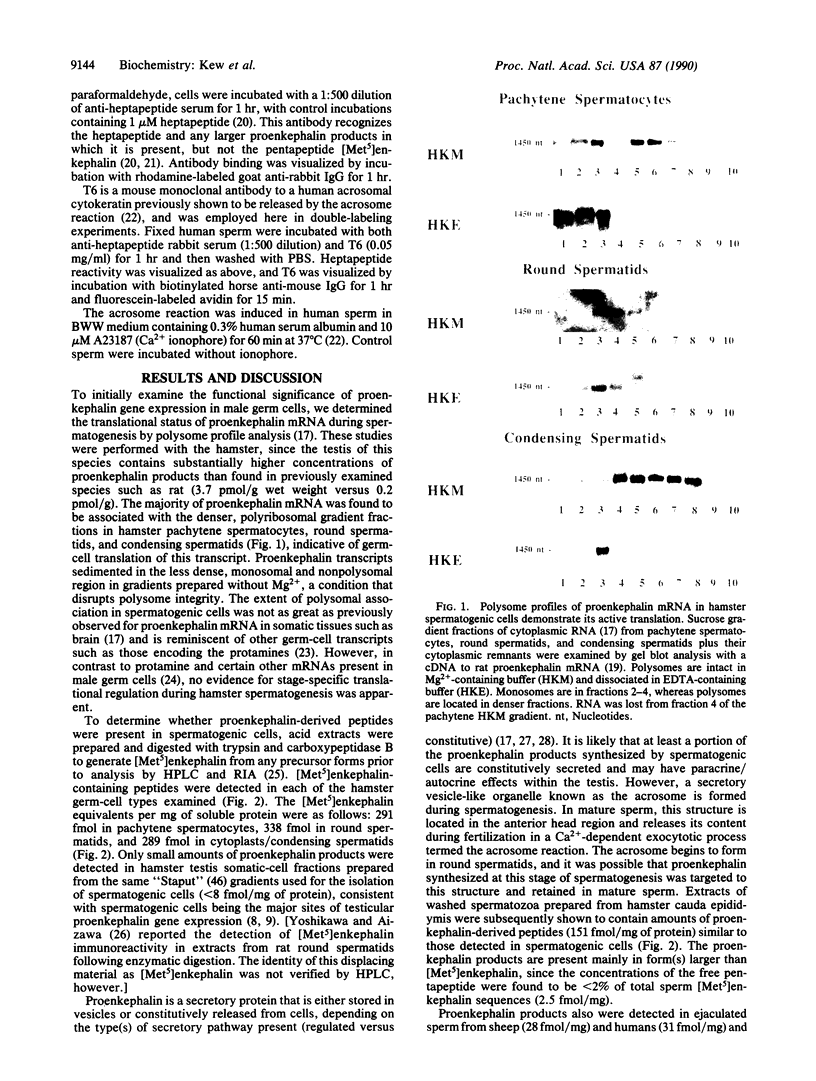
- Kew D., Jin D. F., Kim F., Laddis T., Kilpatrick D. L. Translational status of proenkephalin mRNA in the rat reproductive system. Mol Endocrinol. 1989 Aug;3(8):1191–1196. doi: 10.1210/mend-3-8-1191. [DOI] [PubMed] [Google Scholar]
- Kew D., Kilpatrick D. L. Expression and regulation of the proenkephalin gene in rat Sertoli cells. Mol Endocrinol. 1989 Jan;3(1):179–184. doi: 10.1210/mend-3-1-179. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Borland K., Jin D. F. Differential expression of opioid peptide genes by testicular germ cells and somatic cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5695–5699. doi: 10.1073/pnas.84.16.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Howells R. D., Noe M., Bailey L. C., Udenfriend S. Expression of preproenkephalin-like mRNA and its peptide products in mammalian testis and ovary. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7467–7469. doi: 10.1073/pnas.82.21.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Millette C. F. Expression of proenkephalin messenger RNA by mouse spermatogenic cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5015–5018. doi: 10.1073/pnas.83.14.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Rosenthal J. L. The proenkephalin gene is widely expressed within the male and female reproductive systems of the rat and hamster. Endocrinology. 1986 Jul;119(1):370–374. doi: 10.1210/endo-119-1-370. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Zinn S. A., Fitzgerald M., Higuchi H., Sabol S. L., Meyerhardt J. Transcription of the rat and mouse proenkephalin genes is initiated at distinct sites in spermatogenic and somatic cells. Mol Cell Biol. 1990 Jul;10(7):3717–3726. doi: 10.1128/mcb.10.7.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene K. C., Distel R. J., Hecht N. B. Translational regulation and deadenylation of a protamine mRNA during spermiogenesis in the mouse. Dev Biol. 1984 Sep;105(1):71–79. doi: 10.1016/0012-1606(84)90262-8. [DOI] [PubMed] [Google Scholar]
- Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol Cell Biol. 1989 Nov;9(11):5134–5142. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Selection of initiation sites by eucaryotic ribosomes: effect of inserting AUG triplets upstream from the coding sequence for preproinsulin. Nucleic Acids Res. 1984 May 11;12(9):3873–3893. doi: 10.1093/nar/12.9.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahm H. W., Gerber L. D., Brink L., Kilpatrick D. L., Udenfriend S. Specific polyclonal antibodies to the carboxyl terminus of [Met]enkephalin-Arg6-Gly7-Leu8. Arch Biochem Biophys. 1983 Sep;225(2):422–429. doi: 10.1016/0003-9861(83)90049-8. [DOI] [PubMed] [Google Scholar]
- Lalli M., Clermont Y. Structural changes of the head components of the rat spermatid during late spermiogenesis. Am J Anat. 1981 Apr;160(4):419–434. doi: 10.1002/aja.1001600406. [DOI] [PubMed] [Google Scholar]
- Lewis R. V., Stern A. S., Kimura S., Rossier J., Stein S., Udenfriend S. An about 50,000-dalton protein in adrenal medulla: a common precursor of [Met]- and [Leu]enkephalin. Science. 1980 Jun 27;208(4451):1459–1461. doi: 10.1126/science.7384787. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Morris P. L., Vale W. W., Bardin C. W. Beta-endorphin regulation of FSH-stimulated inhibin production is a component of a short loop system in testis. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1513–1519. doi: 10.1016/s0006-291x(87)80303-0. [DOI] [PubMed] [Google Scholar]
- Olson G. E., Winfrey V. P., Davenport G. R. Characterization of matrix domains of the hamster acrosome. Biol Reprod. 1988 Dec;39(5):1145–1158. doi: 10.1095/biolreprod39.5.1145. [DOI] [PubMed] [Google Scholar]
- Persson H., Rehfeld J. F., Ericsson A., Schalling M., Pelto-Huikko M., Hökfelt T. Transient expression of the cholecystokinin gene in male germ cells and accumulation of the peptide in the acrosomal granule: possible role of cholecystokinin in fertilization. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6166–6170. doi: 10.1073/pnas.86.16.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintar J. E., Schachter B. S., Herman A. B., Durgerian S., Krieger D. T. Characterization and localization of proopiomelanocortin messenger RNA in the adult rat testis. Science. 1984 Aug 10;225(4662):632–634. doi: 10.1126/science.6740329. [DOI] [PubMed] [Google Scholar]
- Rossier J., Liston D., Patey G., Chaminade M., Foutz A. S., Cupo A., Giraud P., Roisin M. P., Henry J. P., Verbanck P. The enkephalinergic neuron: implications of a polyenkephalin precursor. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):393–404. doi: 10.1101/sqb.1983.048.01.043. [DOI] [PubMed] [Google Scholar]
- Roth K. A., Lorenz R. G., Unanue R. A., Weaver C. T. Nonopiate active proenkephalin-derived peptides are secreted by T helper cells. FASEB J. 1989 Oct;3(12):2401–2407. doi: 10.1096/fasebj.3.12.2529160. [DOI] [PubMed] [Google Scholar]
- Stock C. E., Fraser L. R. Divalent cations, capacitation and the acrosome reaction in human spermatozoa. J Reprod Fertil. 1989 Nov;87(2):463–478. doi: 10.1530/jrf.0.0870463. [DOI] [PubMed] [Google Scholar]
- Thomas G., Herbert E., Hruby D. E. Expression and cell type--specific processing of human preproenkephalin with a vaccinia recombinant. Science. 1986 Jun 27;232(4758):1641–1643. doi: 10.1126/science.3754979. [DOI] [PubMed] [Google Scholar]
- Tsong S. D., Phillips D., Halmi N., Liotta A. S., Margioris A., Bardin C. W., Krieger D. T. ACTH and beta-endorphin-related peptides are present in multiple sites in the reproductive tract of the male rat. Endocrinology. 1982 Jun;110(6):2204–2206. doi: 10.1210/endo-110-6-2204. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Kilpatrick D. L. Biochemistry of the enkephalins and enkephalin-containing peptides. Arch Biochem Biophys. 1983 Mar;221(2):309–323. doi: 10.1016/0003-9861(83)90149-2. [DOI] [PubMed] [Google Scholar]
- Viveros O. H., Diliberto E. J., Jr, Hazum E., Chang K. J. Opiate-like materials in the adrenal medulla: evidence for storage and secretion with catecholamines. Mol Pharmacol. 1979 Nov;16(3):1101–1108. [PubMed] [Google Scholar]
- Wolf D. P., Boldt J., Byrd W., Bechtol K. B. Acrosomal status evaluation in human ejaculated sperm with monoclonal antibodies. Biol Reprod. 1985 Jun;32(5):1157–1162. doi: 10.1095/biolreprod32.5.1157. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R., Noda Y. D. Fine structure of the hamster sperm head. Am J Anat. 1970 Jul;128(3):367–388. doi: 10.1002/aja.1001280307. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Aizawa T. Enkephalin precursor gene expression in postmeiotic germ cells. Biochem Biophys Res Commun. 1988 Mar 15;151(2):664–671. doi: 10.1016/s0006-291x(88)80332-2. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Aizawa T. Expression of the enkephalin precursor gene in rat Sertoli cells. Regulation by follicle-stimulating hormone. FEBS Lett. 1988 Sep 12;237(1-2):183–186. doi: 10.1016/0014-5793(88)80197-2. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Aizawa T., Nozawa A. Phorbol ester regulates the abundance of enkephalin precursor mRNA but not of amyloid beta-protein precursor mRNA in rat testicular peritubular cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):568–575. doi: 10.1016/0006-291x(89)92637-5. [DOI] [PubMed] [Google Scholar]