Abstract
Pd-catalyzed meta-C–H chlorination of anilines and phenols is developed using norbornene as the mediator. The presence of heterocycles, including indole, thiophene and indazole, are tolerated. The identification of a new pyridone-based ligand is crucial for the success of this meta-C–H chlorination reaction. Subsequent diverse transformations of the chlorinated products demonstrate the versatility of meta-C–H chlorination.
Graphical abstract
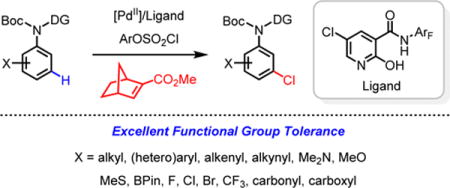
The efficiency and scope of directed ortho-C–H functionalization reactions of arenes has been substantially improved in the past decade.1 In contrast, directed meta-C–H activation remains a significant challenge due to the difficulty of assembling a macrocyclic, cyclophane-like transition state. The use of U-shaped directing templates to direct meta-C–H activation reactions demonstrated the feasibility of overcoming the constraint of distance and geometry. However, the scope of both substrates and transformations remains significantly inferior to ortho-C–H functionalizations.2 The Ru(II) catalyzed meta-C–H functionalization via ortho-cyclometallation and subsequent functionalization at the meta-position is also a promising and elegant approach, though it has only been demonstrated with a small set of substrates thus far.3 Several other approaches towards the meta-C–H functionalization of aromatics have also been reported, though the generality of these approaches remains to be established.4 Recently, directed meta-C–H arylation5 and alkylation5a,5c has been achieved by using norbornene as a transient mediator. In general, a diverse range of meta-C–H functionalizations via this approach remains to be demonstrated. Herein we report the first example of meta-C–H chlorination using this approach. The development of a new pyridone-based ligand is crucial for the success of this reaction. The versatile reactivity of aryl chlorides allows installation of a wide range of functional groups to suit a broad range of synthetic applications. In addition, meta-chlorinated aryls are also found in many FDA approved pharmaceuticals (Fig. 1).6
Figure 1.
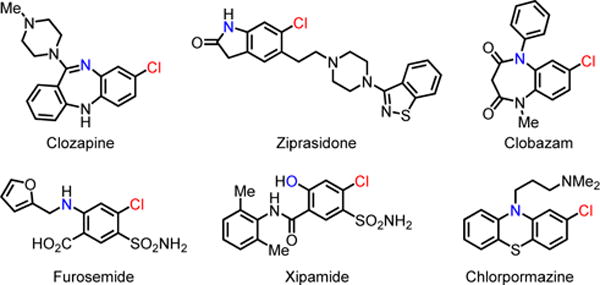
Selected pharmaceuticals containing the meta-chloro aniline or phenol structure.
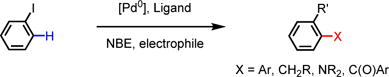
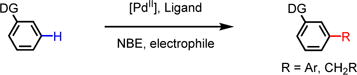
In the Catellani reaction, norbornene is used to relay palladium from the ipso-position to ortho-position of aryl iodides via carbopalladation of the norbornene double bond and subsequent cyclopalladation.7 This reactivity has been exploited to develop a variety of transformations analogous to the Catellani reaction (Eq 1).8 The ability of norbornene to relay palladium from one carbon to the adjacent carbon of an arylpalladium species has recently been exploited to achieve meta-C–H activation reactions initiated by directed ortho-C–H palladation (Eq 2).5 Although the scope of arenes has been substantially expanded by the development of new ligands, many transformations demonstrated in ortho-C–H functionalizations remain to be realized for meta-C–H bonds.5 Given that halides can be converted to various motifs through subsequent transformations, development of meta-C–H halogenation would facilitate the installation of a wide range of functional groups at the meta-position of aromatics. However, the compatibility of the Catellani relay step with halogenation has not been demonstrated to date. In addition, the ortho-C–H palladation in the catalytic cycle of the meta-C–H functionalization could lead to ortho-C–H halogenation with all the well-known C–H halogenating reagents
 |
(eq. 1) |
 |
(eq. 2) |
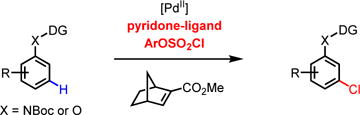 |
(eq. 3) |
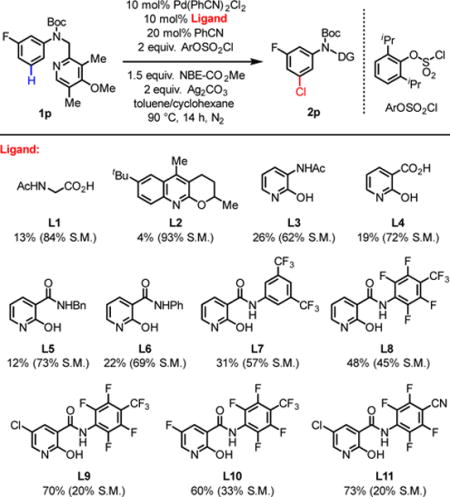
Our initial experimentation used N-chlorosuccinimide (NCS) as the chlorination reagent. Only ortho-chlorinated product was detected under various conditions when using this electrophile.9 These results indicated that NCS reacts with the ortho-aryl palladium(II) intermediates faster than the norbornene. Following this reasoning, we began to search for chlorinating reagents that are less electrophilic, but could still serve as an oxidant in Pd(II)/(IV) catalysis. Among various chlorinating reagents, we found aryl chlorosulfate, initially utilized by the Buchwald group for Pd-catalyzed chlorosulfonylation,10 is potentially suitable for meta-C–H chlorination.11 In the presence of Pd(PhCN)2Cl2, benzonitrile, NBE-CO2Me, and silver carbonate, the use of mono-N-protected amino acid ligand Ac-Gly-OH12 gave the desired product in 13% NMR yield (Table 1). While pyridine L2 is a poor ligand, pyridone derived ligands5e gave promising results (L3–6: 10–30% yields). Since tuning the N-protecting group on L3 did not afford a noticeable improvement (see Supporting Information), we focused on modification of the amide group of L6. Increasing the acidity of the amide was found to improve the yield of the reaction (L7, L8). Chloride substitution at the 5-position of the pyridone further improved the yield to 70% (L9). Fluoride substitution at the same position, however, is less effective (L10). Replacing the CF3 in L9 by a CN group gave a similar result (L11). Other norbornene derivatives gave significantly lower yield (see supporting information).13 Although the necessity of silver carbonate for the formation of the products led us to propose that Ag+ promotes the activation of S–Cl bond of chlorosulfate during the oxidation of palladium(II) intermediate. It is possible that Ag+ also oxidizes Pd(0) or Pd(II) species in the catalytic cycle by a one-electron transfer pathway.
Table 1.
|
Reaction conditions: Pd(PhCN)2Cl2 (10 mol%), Ligand (10 mol%), PhCN (20 mol%), arene 1p (0.1 mmol), 2,6-diiPr-C6H3OSO2Cl (2.0 equiv.), NBE-CO2Me (1.5 equiv.), Ag2CO3 (2.0 equiv.), toluene (1 mL), cyclohexane (1 mL), 90 °C, 14 h, N2.
The yield was determined by 1H NMR analysis of the crude product using 1,1,2,2-tetrachloroethane as an internal standard.
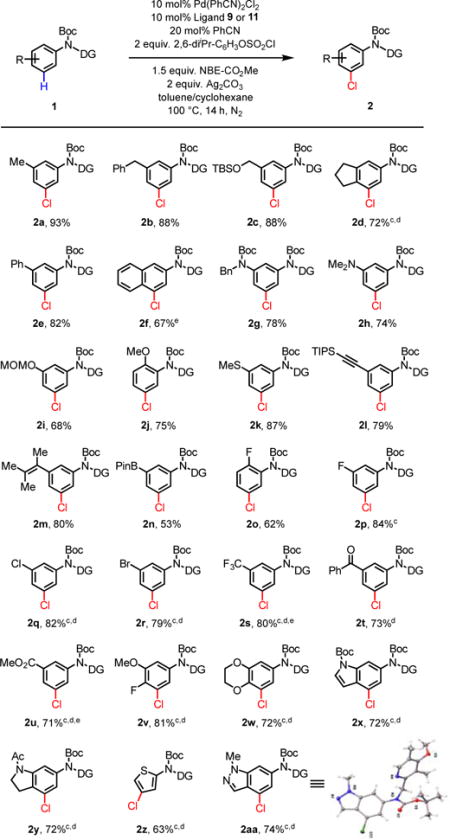
With the optimized ligands (L9 and L11) and reaction conditions in hand, a wide range of aniline substrates were subjected to the standard meta-chlorination conditions (Table 2). Meta-alkylated and arylated anilines gave the meta-chlorinated products in excellent yields (2a–2f). Meta-chlorination of 1a using 5 mol% catalyst also proceeded to give 2a in 72% yield (see supporting information). Meta-chlorination of highly electron-rich arenes containing Me2N, OMe and MeS groups also proceeded smoothly without the formation of side products derived from the electrophilic chlorination (2g–2k). The tolerance of alkynyl and alkenyl groups (2l, 2m) is noteworthy as these functional groups are known to react with electrophilic sources of chlorine, thus demonstrating the mildness and selectivity of this transformation. Intriguingly, aryl borate remained largely intact in the presence of silver salts during the meta-chlorination (2n). Various electron-withdrawing groups including fluoro (2o, 2p), chloro (2q), bromo (2r), trifluoromethyl (2s), carbonyl (2t), and carboxyl (2u) are also compatible with the reaction. A number of medicinally important heterocyclic substrates were also smoothly converted to the meta-chlorinated products 2w–2aa in good yields. Hydroxyl group and pyridines are not compatible with current reaction conditions. Unsubstituted aniline substrate, 1ab, was converted to the di-substituted product in 77% yield (Eq 4). The feasibility of extending this protocol to phenol substrates was also demonstrated with modified conditions (Eq 5). A gram-scale reaction was conducted and the product 2a was obtained in 87% yield (Eq 6).
Table 2.
|
Reaction conditions: Pd(PhCN)2Cl2 (10 mol%), Ligand 9 (10 mol%), PhCN (20 mol%), arene 1 (0.1 mmol), 2,6-diiPr-C6H3OSO2Cl (2.0 equiv.), NBE-CO2Me (1.5 equiv.), Ag2CO3 (2.0 equiv.), toluene (1 mL), cyclohexane (1 mL), 100 °C, 14 h, N2.
Isolated yield.
110 °C.
Ligand L11.
Pd(PhCN)2Cl2 (15 mol%), Ligand (15 mol%).
To demonstrate the utility of this meta-chlorination reaction, we performed diversification of 2a using the meta-chloro group as a synthetic handle (Scheme 1). Aniline 2a was converted into a wide range of desirable synthons in good yields by known methods including amination14, cross-coupling15, α-arylation16, borylation17, methoxylation18, and alkynylation19. Notably, none of the functional groups, shown in scheme 1, can currently be installed through previously reported norbornene-mediated meta-C–H activation methods. Furthermore, the pyridine directing group was readily removed by treatment with hydrobromic acid in excellent yield (see supporting information for details).
 |
(eq. 4) |
 |
(eq. 5) |
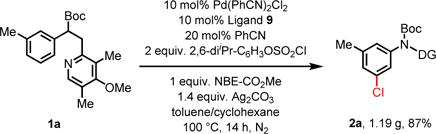 |
(eq. 6) |
Scheme 1. Diversification of meta-Chloro Aniline.
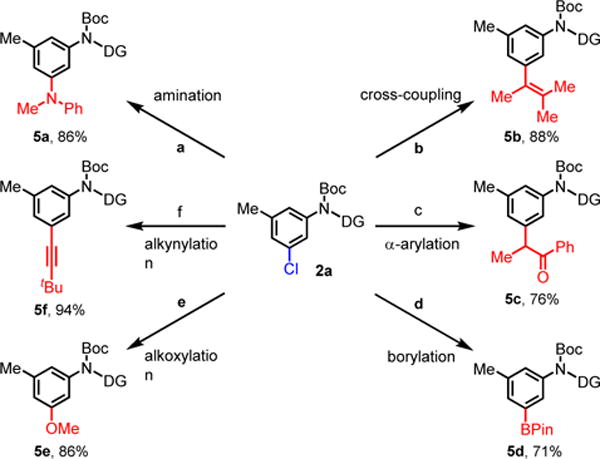
Reaction conditions: aPd2(dba)3 (3 mol%), DavePhos (9 mol%), PhMeNH (1.5 equiv.), arene 2a (0.1 mmol), NaOtBu (1.4 equiv.), toluene (0.4 mL), 80 °C. bPd(OAc)2 (4 mol%), XPhos (8 mol%), 3-Methyl-2-buten-2-ylboronic acid pinacol ester (2.0 equiv.), arene 2a (0.1 mmol), K3PO4 (3.0 equiv.), THF (1 mL), 60 °C. cPd(OAc)2 (5 mol%), MePhos (10 mol%), propiophenone (1.2 equiv.), arene 2a (0.1 mmol), NaOtBu (1.3 equiv.), toluene (0.2 mL), 80 °C. dPd(OAc)2 (5 mol%), XPhos (10 mol%), B2Pin2 (2.0 equiv.), arene 2a (0.1 mmol), KOAc (3.0 equiv.), dioxane (0.4 mL), 110 °C. ePd2(dba)3 (3 mol%), tBuXPhos (12 mol%), MeOH (10 equiv.), arene 2a (0.1 mmol), NaOtBu (1.4 equiv.), dioxane (0.4 mL), 100 °C. fPd(MeCN)2Cl2 (6 mol%), XPhos (18 mol%), tert-butylacetylene (1.4 equiv.), arene 2a (0.1 mmol), Cs2CO3 (2.5 equiv.), MeCN (0.4 mL), 90 °C.
To gain insights into the two C–H activation steps of the catalytic cycle, we measured the parallel kinetic isotope effects (KIE) (Scheme 2). The results indicate that neither ortho- nor meta-C–H cleavage is the rate-limiting step. The competition experiments reveal that electron neutral substrate (1a) is more reactive than both electron rich and deficient substrates (1ac, 1t).
Scheme 2.
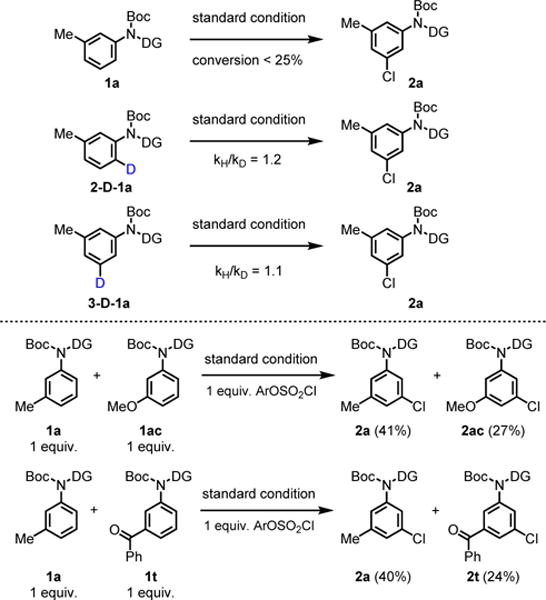
KIE Studies and Competition Experiments.
Based on the previous work by our group and others, a proposed catalytic cycle for the norbornene mediated meta-chlorination is depicted in scheme 3. Key to the success of this catalytic cycle is selective oxidation of palladacycle b by the chlorosulfate. Intermediate b arises from intermediate a through norbornene insertion and metalation. We hypothesize that the oxidation of b produces Pd(IV) intermediate c through palladium insertion into the S–Cl bond of the aryl chlorosulfate. Reductive elimination from this proposed intermediate would form the C–Cl bond at the meta-position. Meanwhile, sulfate on palladium (intermediate c) could be degraded into sulfur dioxide and phenol.11,20 Norbornene is then released via β-carbon elimination, which generates intermediate d. Protodemetalation of the aryl–palladium bond yields the final product 2 and regenerates the palladium catalyst.
Scheme 3.
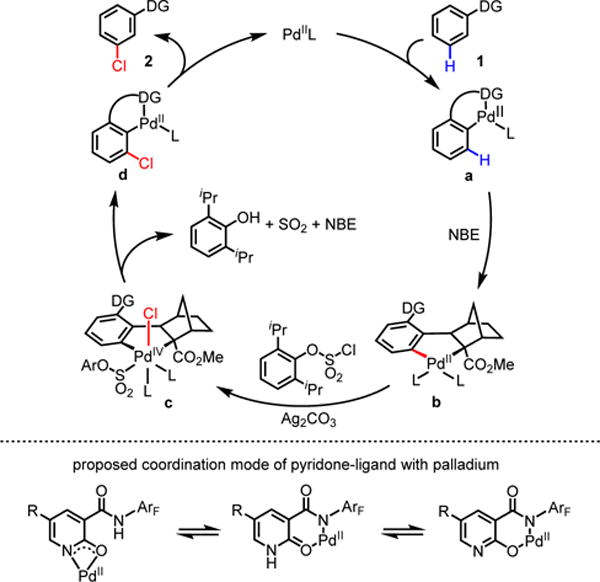
Proposed Mechanism.
In summary, we have developed the first example of directed meta-selective C(sp2)–H chlorination of arenes. The reaction, promoted by a new pyridone ligand, displays outstanding functional group tolerance. Meta-C(sp2)–H chlorination of other classes of substrates will be reported in due course.
Supplementary Material
Acknowledgments
We gratefully acknowledge The Scripps Research Institute and NIH (NIGMS, 2R01 GM102265).
Footnotes
Supporting Information Available: Experimental procedures and spectral data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For selected reviews on transition metal catalyzed C–H activation, see:; (a) Chen X, Engle KM, Wang D-H, Yu J-Q. Angew Chem Int Ed. 2009;48:5094. doi: 10.1002/anie.200806273. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lyons TW, Sanford MS. Chem Rev. 2010;110:1147. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Daugulis O, Do HQ, Shabashov D. Acc Chem Res. 2009;42:1074. doi: 10.1021/ar9000058. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Colby DA, Bergman RG, Ellman JA. Chem Rev. 2010;110:624. doi: 10.1021/cr900005n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Arockiam PB, Bruneau C, Dixneuf PH. Chem Rev. 2012;112:879. doi: 10.1021/cr300153j. [DOI] [PubMed] [Google Scholar]; (f) Kuhl N, Hopkinson MN, Wencel-Delord J, Glorius F. Angew Chem Int Ed. 2012;51:10236. doi: 10.1002/anie.201203269. [DOI] [PubMed] [Google Scholar]
- 2.For selected examples of template directed meta-C–H functionalization, see:; (a) Leow D, Li G, Mei TS, Yu JQ. Nature. 2012;486:518. doi: 10.1038/nature11158. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tang R, Li G, Yu JQ. Nature. 2014;507:215. doi: 10.1038/nature12963. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wan L, Dastbaravardeh N, Li G, Yu JQ. J Am Chem Soc. 2013;135:18056. doi: 10.1021/ja410760f. [DOI] [PMC free article] [PubMed] [Google Scholar]; For examples of template directed para-C–H functionalization, see:; (d) Bag S, Patra T, Modak A, Deb A, Maity S, Dutta U, Dey A, Kancherla R, Maji A, Hazra A, Bera M, Maiti D. J Am Chem Soc. 2015;137:11888. doi: 10.1021/jacs.5b06793. [DOI] [PubMed] [Google Scholar]; (e) Patra T, Bag S, Kancherla R, Mondal A, Dey A, Pimparkar S, Agasti S, Modak A, Maiti D. Angew Chem, Int Ed. 2016;55:7751. doi: 10.1002/anie.201601999. [DOI] [PubMed] [Google Scholar]
- 3.For examples of Ru(II) catalyzed meta-C–H functionalization via ortho-cyclometallation, see:; (a) Saidi O, Marafie J, Ledger AEW, Liu PM, Mahon MF, Kociok-Köhn G, Whittlesey MK, Frost CG. J Am Chem Soc. 2011;133:19298. doi: 10.1021/ja208286b. [DOI] [PubMed] [Google Scholar]; (b) Hofmann N, Ackermann L. J Am Chem Soc. 2013;135:5877. doi: 10.1021/ja401466y. [DOI] [PubMed] [Google Scholar]; (c) Li J, Warratz S, Zell D, De Sarkar S, Ishikawa EE, Ackermann L. J Am Chem Soc. 2015;137:13894. doi: 10.1021/jacs.5b08435. [DOI] [PubMed] [Google Scholar]; (d) Teskey CJ, Lui AYW, Greaney MF. Angew Chem Int Ed. 2015;54:11677. doi: 10.1002/anie.201504390. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Paterson AJ, StJohn-Campbell S, Mahon MF, Press NJ, Frost CG. Chem Comm. 2015;51:12807. doi: 10.1039/c5cc03951g. [DOI] [PubMed] [Google Scholar]; (f) Fan Z, Ni J, Zhang A. J Am Chem Soc. 2016;138:8470. doi: 10.1021/jacs.6b03402. [DOI] [PubMed] [Google Scholar]
- 4.For selected examples using copper and aryl iodoniums to achieve meta-C–H arylation, see:; (a) Phipps RJ, Gaunt MJ. Science. 2009;323:1593. doi: 10.1126/science.1169975. [DOI] [PubMed] [Google Scholar]; (b) Duong HA, Gilligan RE, Cooke ML, Phipps RJ, Gaunt MJ. Angew Chem Int Ed. 2010;50:463. doi: 10.1002/anie.201004704. [DOI] [PubMed] [Google Scholar]; (c) Yang Y, Li R, Zhao Y, Zhao D, Shi Z. J Am Chem Soc. 2016;138:8734. doi: 10.1021/jacs.6b05777. [DOI] [PubMed] [Google Scholar]; For an example of using CO2 as a traceless directing group, see:; (d) Luo J, Preciado S, Larrosa I. J Am Chem Soc. 2014;136:4109. doi: 10.1021/ja500457s. [DOI] [PubMed] [Google Scholar]; For an example using deprotonation, see:; (e) Martinez-Martinez AJ, Kennedy AR, Mulvey RE, O’Hara CT. Science. 2014;346:834. doi: 10.1126/science.1259662. [DOI] [PubMed] [Google Scholar]
- 5.(a) Wang XC, Gong W, Fang LZ, Zhu RY, Li S, Engle KM, Yu JQ. Nature. 2015;519:334. doi: 10.1038/nature14214. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dong Z, Wang J, Dong G. J Am Chem Soc. 2015;137:5887. doi: 10.1021/jacs.5b02809. [DOI] [PubMed] [Google Scholar]; (c) Shen PX, Wang XC, Wang P, Zhu RY, Yu JQ. J Am Chem Soc. 2015;137:11574. doi: 10.1021/jacs.5b08914. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Han J, Zhang L, Zhu Y, Zheng Y, Chen X, Huang ZB, Shi DQ, Zhao Y. Chem Comm. 2016;52:6903. doi: 10.1039/c6cc02384c. [DOI] [PubMed] [Google Scholar]; (e) Wang P, Farmer ME, Huo X, Jain P, Shen PX, Ishoey M, Bradner JE, Wisniewski SR, Eastgate MD, Yu JQ. J Am Chem Soc. 2016;138:9269. doi: 10.1021/jacs.6b04966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For an example of direct chlorination of (hetero)arenes, see:; (a) Rodriguez RA, Pan CM, Yabe Y, Kawamata Y, Eastgate MD, Baran PS. J Am Chem Soc. 2014;136:6908. doi: 10.1021/ja5031744. [DOI] [PMC free article] [PubMed] [Google Scholar]; For selected reviews on chlorine containing biologically active molecules, see:; (b) Naumann K. Pest Manag Sci. 2000;56:3. [Google Scholar]; (c) Hernandes MZ, Cavalcanti SMT, Moreira DRM, de Azevedo WF, Leite ACL. Curr Drug Targets. 2010;11:303. doi: 10.2174/138945010790711996. [DOI] [PubMed] [Google Scholar]
- 7.(a) Bocelli G, Catellani M, Chiusoli GP, Larocca S. J Organomet Chem. 1984;265:C9. [Google Scholar]; (b) Bocelli G, Catellani M, Chiusoli GP. J Organomet Chem. 1984;279:225. [Google Scholar]
- 8.For reviews of the Catellani reaction, see:; (a) Martin SA, Mariampillai B, Lautens M. Top Curr Chem. 2010;2921:1. doi: 10.1007/128_2009_13. [DOI] [PubMed] [Google Scholar]; (b) Ferraccioli R. Synthesis. 2013;45:581. [Google Scholar]; (c) Catellani M, Motti E, Della Ca’ N. Acc Chem Res. 2008;41:1512. doi: 10.1021/ar800040u. [DOI] [PubMed] [Google Scholar]; (d) Catellani M. Synlett. 2003:298. [Google Scholar]; (e) Catellani M. Top Organomet Chem. 2005;14:21. [Google Scholar]; (f) Chiusoli GP, Catellani M, Costa M, Motti E, Della Ca’ N, Maestri G Coord. Chem Rev. 2010;254:456. [Google Scholar]; (g) Della Ca’ N, NiFontana M, Motti E, Catallani M. Acc Chem Res. 2016;49:1389. doi: 10.1021/acs.accounts.6b00165. [DOI] [PubMed] [Google Scholar]
-
9.Ortho-chlorination with NCS:
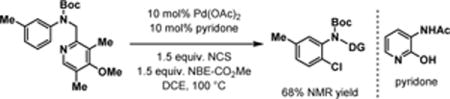
- 10.Debergh JR, Niljianskul N, Buchwald SL. J Am Chem Soc. 2013;135:10638. doi: 10.1021/ja405949a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.For an example using arylsulfonyl chloride to achieve othro-C–H sulfonylation and chlorination, see:; Zhao X, Dimitrijević E, Dong VM. J Am Chem Soc. 2009;131:3466. doi: 10.1021/ja900200g. [DOI] [PubMed] [Google Scholar]
- 12.(a) Wang DH, Engle KM, Shi BF, Yu JQ. Science. 2010;327:315. doi: 10.1126/science.1182512. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Engle KM, Wang DH, Yu JQ. J Am Chem Soc. 2010;132:14137. doi: 10.1021/ja105044s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Musaev DG, Kaledinm AL, Shi BF, Yu JQ. J Am Chem Soc. 2012;134:1690. doi: 10.1021/ja208661v. [DOI] [PubMed] [Google Scholar]; (d) Cheng Gu-J, Yang Y-F, Liu P, Chen P, Sun T-Y, Li G, Zhang X, Houk KN, Yu J-Q, Wu Y-D. J Am Chem Soc. 2014;136:894. doi: 10.1021/ja411683n. [DOI] [PubMed] [Google Scholar]
- 13.Other directing groups are not compatible with this catalytic cycle, affording no desired products
- 14.Old DW, Wolfe JP, Buchwald SL. J Am Chem Soc. 1998;120:9722. [Google Scholar]
- 15.Barder TE, Walker SD, Martinelli JR, Buchwald SL. J Am Chem Soc. 2005;127:4685. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]
- 16.Fox JM, Huang X, Chieffi A, Buchwald SL. J Am Chem Soc. 2000;122:1360. [Google Scholar]
- 17.Billingsley KL, Barder TE, Buchwald SL. Angew Chem, Int Ed. 2007;46:5359. doi: 10.1002/anie.200701551. [DOI] [PubMed] [Google Scholar]
- 18.Cheung CW, Buchwald SL. Org Lett. 2013;15:3998. doi: 10.1021/ol401796v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelman D, Buchwald SL. Angew Chem, Int Ed. 2003;42:5993. doi: 10.1002/anie.200353015. [DOI] [PubMed] [Google Scholar]
-
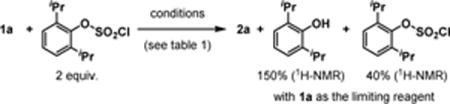
20.2,6-Diisopropylphenol was observed as the product from aryl chlorosulfate:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


