Abstract
Oligodendrocytes are generated from oligodendrocyte precursor cells (OPCs). Mechanisms of OPC differentiation have been extensively examined with two-dimensional cell culture systems. However, these cellular events may be more accurately represented using a three-dimensional (3D) model. In this study, we report the development of a novel 3D OPC culture system using gels composed of a mixture of collagen and hyaluronan, wherein cultured rat primary OPCs can proliferate and differentiate into oligodendrocytes. Our data show that the gel concentration and cell-seeding density are critical factors for the numbers of OPCs and oligodendrocytes in our 3D culture system. In addition, Notch signaling, which supports cell-to-cell communication, may also be important for OPC function in our system because a Notch inhibitor DAPT suppressed OPC proliferation and differentiation. Taken together, cultured rat OPCs can grow in collagen-/hyaluronan-based gels, and our novel 3D OPC culture system may offer a useful platform for examining the mechanisms of OPC function in vitro.
Keywords: : oligodendrocyte precursor cell, astrocyte, 3D culture, Notch signaling, cell–cell interaction
Introduction
Oligodendrocyte precursor cells (OPCs) comprise the primary source of oligodendrocytes that form myelin sheaths around axons in the central nervous system. Proper regulation of OPC-to-oligodendrocyte differentiation is necessary to maintain a healthy oligodendrocyte population for effective axon ensheathing. Mechanisms of OPC differentiation have been extensively examined with in vitro cell culture models in two-dimensional (2D) culture systems. However, these cellular events may be more accurately represented in three-dimensional (3D) conditions. Therefore, 3D OPC culture systems need to be developed to understand OPC function more precisely.
Compared to 2D culture conditions, a 3D system offers more opportunities to model the physical parameters that determine cell–cell interactions. In a 3D system, each cell can interact with a larger number of neighboring cells, and possibly with multiple cells simultaneously. Cell–cell interaction is of paramount importance in the regulation of cell proliferation and differentiation in the central nervous system [1]. For example, in 3D culture conditions using biomaterial-based gels, a specific vessel structure can arise from the cell–cell interaction of cultured endothelial cells through self-organization [2]. In addition, some pathogenic processes of specific protein accumulation can also be enhanced in 3D conditions [3]. Therefore, the cell–cell contact in 3D culture systems could shed new light on the processes that maintain cellular function and survival.
Thus far, however, there have been no reports in the literature in which OPC function in vitro is examined using 3D conditions in biomaterial-based gels. Hence, we aimed to develop a novel 3D culture platform to observe the proliferation and differentiation of OPCs in vitro. In this study, we used collagen-/hyaluronan-mixed gels as a scaffolding biomaterial for OPC cultures. Seeded OPCs were viable in the gel and successfully differentiated into oligodendrocytes over time. Our novel 3D OPC culture system offers a useful platform for examining the mechanisms of OPC function.
Materials and Methods
All experiments were performed following institutionally approved protocols by Massachusetts General Hospital Subcommittee on Research Animal Care, and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
OPC isolation
Primary cortical OPCs were prepared for experiments according to our previous work [4,5]. Briefly, primary mixed glial cells were obtained from the brains of postnatal day 1 Sprague-Dawley (SD) rats and cultured in Dulbecco's modified Eagle's medium (DMEM; ThermoFisher, #11965) containing 20% fetal bovine serum. Ten days later, the flasks were shaken for 1 h on an orbital shaker (218 rpm) at 37°C to remove microglia. They were then changed to a new medium and shaken overnight (∼20 h). The medium was collected and plated on noncoated tissue culture dishes for 1 h at 37°C to remove contaminating astrocytes and microglia. Finally, the nonadherent cells in the culture media were used as OPCs in this study.
3D culture of OPC
The Hystem®-C (#GS1005, ESI-BIO) reagent kit, which contains Gelin-S®, Gycosil®, and Extralink®, was used to construct the 3D hydrogel. The suitability of this hydrogel is based, in part, on prior work, which demonstrated that CD44, the receptor for hyaluronan, is required for the migration of transplanted OPCs in a rat model of spinal cord injury [6]. Experiments from another group have also demonstrated that many cell types bind to denatured collagen type I (i.e., collagen) through tripeptide arg-gly-asp (RGD) sites [7], and differentiated OPCs formed significantly more myelinated nanofiber segments when collagen was incorporated into the fibrous substrate in which they were growing [8]. For this study, isolated OPCs described above were mixed with a gel mixture containing Gelin-S (Thiol-modified collagen), Gycosil (Thiol-modified hyaluronan), and Extralink (Thiol-reactive PEGDA crosslinker) (in a ratio of 2:2:1). The viscosity of gels was altered according to manufacturer's protocol. Standard condition of gel viscosity was defined as a mixture of 1:12 (gel to culture medium). The gel mixture containing isolated OPCs was then passaged onto the well of 12-well culture plates (CELLSTAR, greiner bio-one: 1 mL culture medium per well) after vortexing. The cell density of standard conditions was 3,600 cells/mL. For the first 3 days, cells were maintained in the proliferation medium. Then, the cell culture medium was removed, and the differentiation medium was added to the culture wells to initiate OPC differentiation into mature oligodendrocytes. The proliferation media consisted of Neurobasal medium (#21103, ThermoFisher) containing 1% penicillin/streptomycin (#10378, ThermoFisher), 2 mM glutamine (#25030, ThermoFisher), 2% B27 supplement (#17504, ThermoFisher), 10 ng/mL basic fibroblast growth factor (FGF) (#100-18, Peprotech), and 10 ng/mL platelet-derived growth factor-AA (PDGF-AA) (#100-13, Peprotech). The differentiation media consisted of DMEM containing 1% penicillin/streptomycin, 2% B27 supplement, 10 ng/mL ciliary neurotrophic factor (CNTF) (#450-50, Peprotech), and 15 nM T3 (#T6397, Sigma).
Immunocytochemistry
On day 1, 3, or 10 after passage, cells were fixed using 4% PFA for 15 min. After being washed thrice in phosphate-buffered saline (PBS) containing 0.1% Triton X-100, they were incubated with 1% bovine serum albumin in PBS for 1 h. Cells were immunostained with primary antibodies of anti-platelet-derived growth factor receptor α (PDGF-Rα) (OPC marker; 1:100; R&D, #AF1062), anti-glutathione-S-transferase (GST)-pi (oligodendrocyte marker; 1:200; MBL, #312), and GFAP (astrocyte marker; 1:200; Invitrogen, #18-0063). For counting the cell number, Z-stacked images were randomly captured and cell counting was performed in a double-blinded manner by megascopic counting of labeled cells from at least three wells of each condition. For 3D reconstruction imaging on OPC culture, Z-stack imaging was performed with a 20 × objective using the corresponding lasers. Briefly, the procedure was to set up 1 μm step size and then consecutive cross-sectional images (XYZ) were acquired from top to bottom of appropriate images. After acquisition, 3D reconstruction and display of cubic imaging were constructed by 3D Olympus Fluoview software.
Quantitative real-time reverse transcription-PCR
RNA of cultured cells in our 3D culture system was isolated with the RNeasy Plus Mini kit (QIAGEN, Hilden, Germany), and first-strand cDNA was synthesized with PrimeScript RT reagent (Takara-Clonetech). Quantitative real-time reverse transcription-PCR (QRT-PCR) was performed using SYBR Premix Ex TaqII (Takara-Clonetech) and analyzed with Fast real-time system 7500 (Applied Biosystems). We used shuttle polymerase chain reaction protocol (95°C, 30 s, repeat 1 for initial denaturation and 95°C, 3 s and 60°C, 30 s, repeat >40 for cycling). We measured the volume of synthesized RNA using Nanodrop (Thermo) and confirmed the validity of GAPDH as housekeeping gene, which is stably expressed in OPC cultures [9]. We prepared a series of fourfold dilution for relative standard curve and checked the functional quality of each primer by validating the standard curves in every run. Then, we quantified the expression level of target gene relative to GAPDH as reference gene. Expression levels were measured relative to GAPDH as internal control. The sequences of primers used in this study are as follows; 5′-TCCAGTATGACTCTACCCACG-3′ for rat GAPDH forward; 5′-CACGACATACTCAGCACCAG-3′ for rat GAPDH reverse; 5′-TGTGGACTCTGACAACGCGTACAT-3′ for rat PDGF-Rα forward; 5′-ATCTCTGTTCATCCAGGCCACCTT-3′ for rat PDGF-Rα reverse; 5′-TTGACTCCATCGGGCGCTTCTTTA-3′ for rat MBP forward; 5′-TTCATCTTGGGTCCTCTGCGACTT-3′ for rat MBP reverse; 5′-GAGATGATGGAGCTCAATGACC-3′ for rat GFAP forward; and 5′-CTGGATCTCCTCCTCCAGCGA-3′ for rat GFAP reverse.
Cellular viability assay
Cellular viability was quantified at 3 days after cell passage by a standard measurement of lactate dehydrogenase (LDH) using the LDH assay kit (#11644793001, Roche).
Gel permeability assay
OPCs were passaged onto transwells coated with a gel mixture of gel with different concentrations (12 mm diameter, 3.0 μm pore size polycarbonate filter, Corning). Fluorescein isothiocyanate-labeled dextran (molecular weight, 40,000; Sigma, FD40) was added to the upper chamber. After 24-h incubation, 100 μL of culture media from the lower compartment was collected and measured for fluorescence (excitation 488 nm, emission 516 nm) using a spectrophotometer.
Statistical analysis
Statistical significance was evaluated using the unpaired t-test (or Welch's t-test if the normality of distribution was not assumed) to compare differences between the two groups and a one-way ANOVA followed by Tukey's honestly significant difference test for multiple comparisons. Data are expressed as mean ± standard deviation. A P-value of <0.05 was considered statistically significant.
Results
OPCs proliferate and differentiate into oligodendrocytes in 3D gels
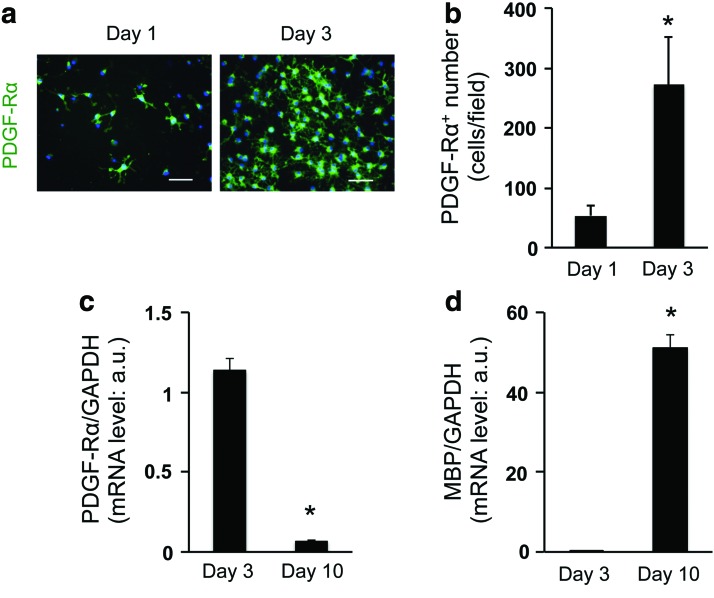
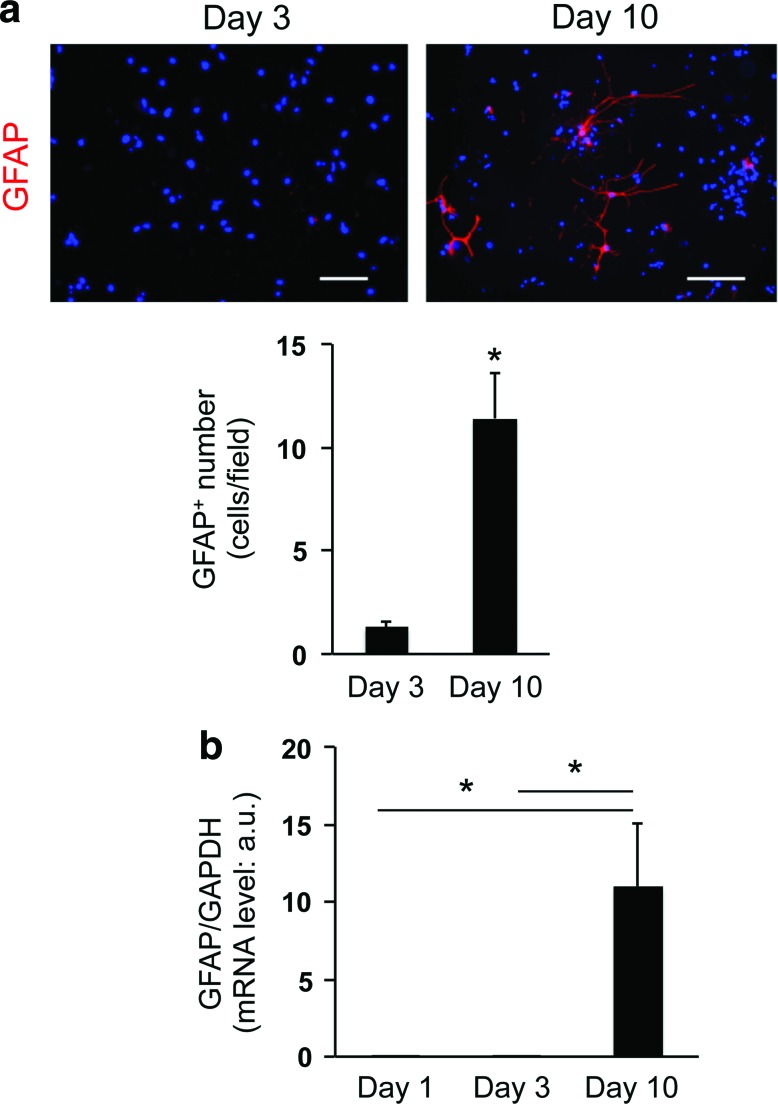
To develop the 3D OPC culture system, we selected collagen/hyaluronan gels as scaffolding biomaterial, which is easy to handle and does not contain any growth factors. The absence of growth factors is important because the process of OPC proliferation/differentiation would be affected by these regulatory proteins. In this study, OPCs were collected by shaking mixed glial cultures derived from neonatal rat cortex. Our previous reports confirmed that in the standard 2D culture system, those cultured OPCs from rat neonatal cortex showed several OPC marker proteins, including PDGF-Rα, and differentiated into mature oligodendrocytes over time [10,11]. In this study, we maintained OPC cultures in the standard OPC proliferation medium during the first 3 days after cell seeding, after which the culture medium was switched to the differentiation medium. Immunostaining analysis showed that compared to day 1, the number of PDGF-Rα-positive OPCs is higher on day 3 (Fig. 1a, b, Supplementary Fig. S1, and Supplementary Video S1; Supplementary Data are available online at www.liebertpub.com/scd), suggesting that OPCs successfully proliferated in the 3D conditions. On the other hand, the level of PDGF-Rα mRNA dramatically dropped after a 7-day period of differentiation (e.g., from day 3 to 10; Fig. 1c), after which time, a high level of MBP mRNA was seen (Fig. 1d). Notably, during the 7-day differentiation period, a small, but detectable number of GFAP-positive astrocytes appeared (Fig. 2a–c), indicating that our 3D OPC culture system may mimic the minor OPC-to-astrocyte conversion observed in vivo [12,13].
FIG. 1.
Cultured OPCs proliferate and differentiate into oligodendrocyte in three-dimensional (3D) gels: (a) representative image for PDGF-Rα staining of cells on day 1 (left) and day 3 (right). Scale bar: 100 μm. (b) Cell numbers for PDGF-Rα-positive cells on day 1 and day 3. *P < 0.01 of N = 5, mean ± SD. (c, d) mRNA levels of PDGF-Rα (c) and MBP (d) on day 3 and 10. *P < 0.01 of N = 4, mean ± SD. Please see Supplementary Fig. S1 and Supplementary Video S1 for 3D images of our OPC culture. OPC, oligodendrocyte precursor cell; PDGF-Rα, platelet-derived growth factor receptor α; SD, standard deviation. Color images available online at www.liebertpub.com/scd
FIG. 2.
Minor conversion from OPCs to astrocytes in 3D culture system: (a) representative image for GFAP staining of cells on day 3 (left) and day 10 (right). Scale bar: 100 μm. Cell numbers for GFAP-positive cells on day 3 and 10. *P < 0.01 of N = 5, mean ± SD. (b) mRNA levels of GFAP on day 1, 3, and 10. *P < 0.01 of N = 4, mean ± SD. Color images available online at www.liebertpub.com/scd
Gel concentration and cell-seeding density are critical factors for OPC function
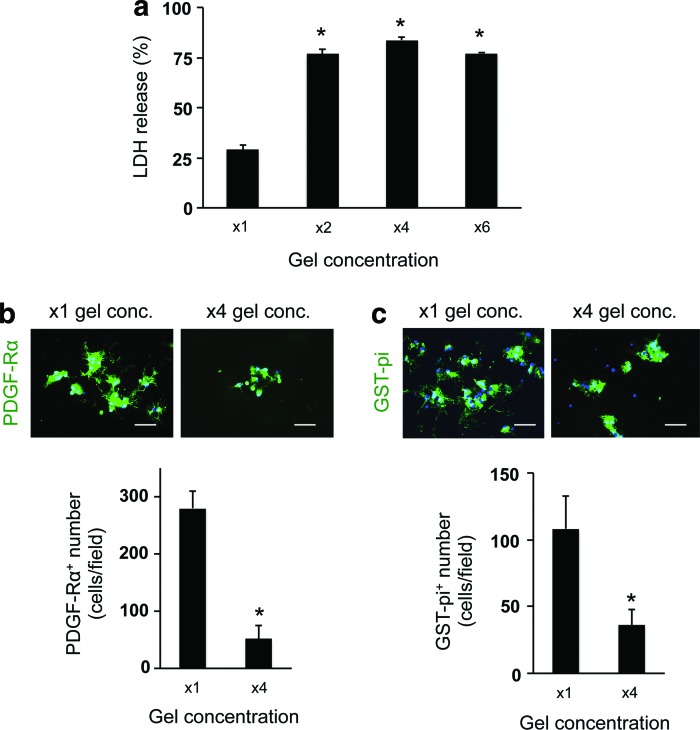
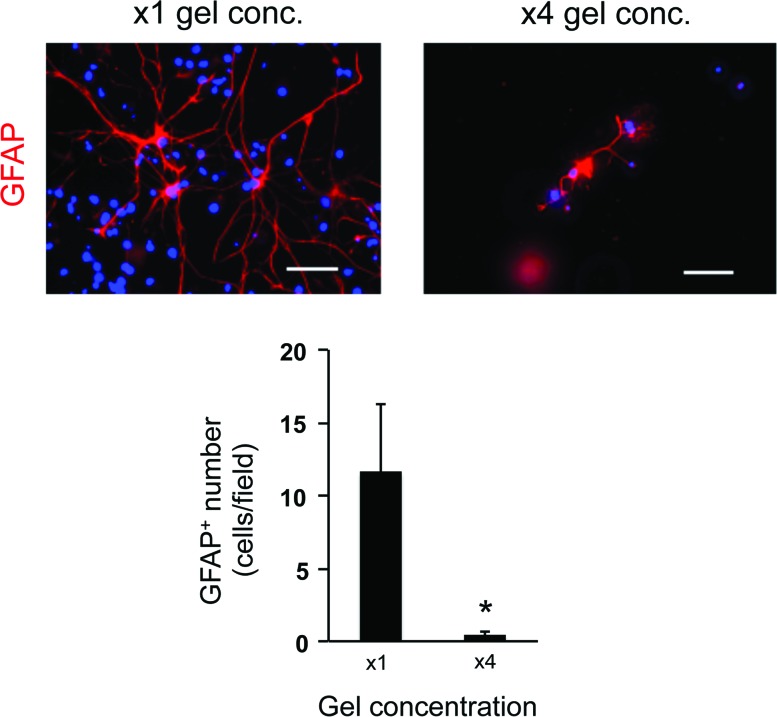
To optimize the culture conditions for OPCs in the 3D culture system, we next changed the gel concentration and/or cell-seeding density. The gel concentration was modulated by changing the ratio of gel to medium. In our study, we prepared four conditions of gel concentration—control conditions (x1 gel concentration: e.g., the ratio to culture media was 1:12, according to the manufacture's protocol suggested), x2 gel concentration, x4 gel concentration, and x6 gel concentration. The LDH assay showed that cells in higher gel concentration (e.g., higher gel viscosity) exhibited worse cellular conditions (Fig. 3a). Concomitantly, the numbers of OPCs on day 3 and oligodendrocytes on day 10 in the higher gel concentration conditions were lower than the ones in the control conditions (Fig. 3b, c), indicating that the increase of gel concentration/viscosity may reduce OPC differentiation as well as OPC survival. Consequently, the number of GFAP-positive astrocytes was similarly decreased due to the decreased number of OPCs (Fig. 4). However, the gel thickness of our 3D culture system was not significantly altered by changing the gel concentration: x1 gel concentration: 153 ± 66 μm thickness and x4 gel concentration: 168 ± 57 μm. In addition, the permeability of FITC-dextran (40 kDa) for the hydrogel was similar between the x1 and x4 gel concentration conditions (Supplementary Fig. S2), indicating that, regardless of gel concentration, cells would receive sufficient nutrients from culture media in our 3D culture system.
FIG. 3.
Oligodendrocyte lineage cells in low porosity gels: (a) LDH assay showed that cells in the lower porosity gels showed higher LDH release amount on day 3. *P < 0.01 of N = 4, mean ± SD. (b) PDGF-Rα staining on day 3. The lower porosity conditions showed less number of PDGF-Rα-positive cells on day 3. *P < 0.01 of N = 5, mean ± SD. Scale bar: 100 μm. (c) GST-pi staining on day 10. The lower porosity conditions showed less number of GST-pi-positive cells on day 10. *P < 0.01 of N = 4–5, mean ± SD. Scale bar: 100 μm. GST, glutathione-S-transferase; LDH, lactate dehydrogenase. Color images available online at www.liebertpub.com/scd
FIG. 4.
Astrocytes in low porosity gels: GFAP staining on day 10. The lower porosity conditions showed less number of GFAP-positive cells on day 10. *P < 0.01 of N = 5, mean ± SD. Scale bar: 100 μm. Color images available online at www.liebertpub.com/scd
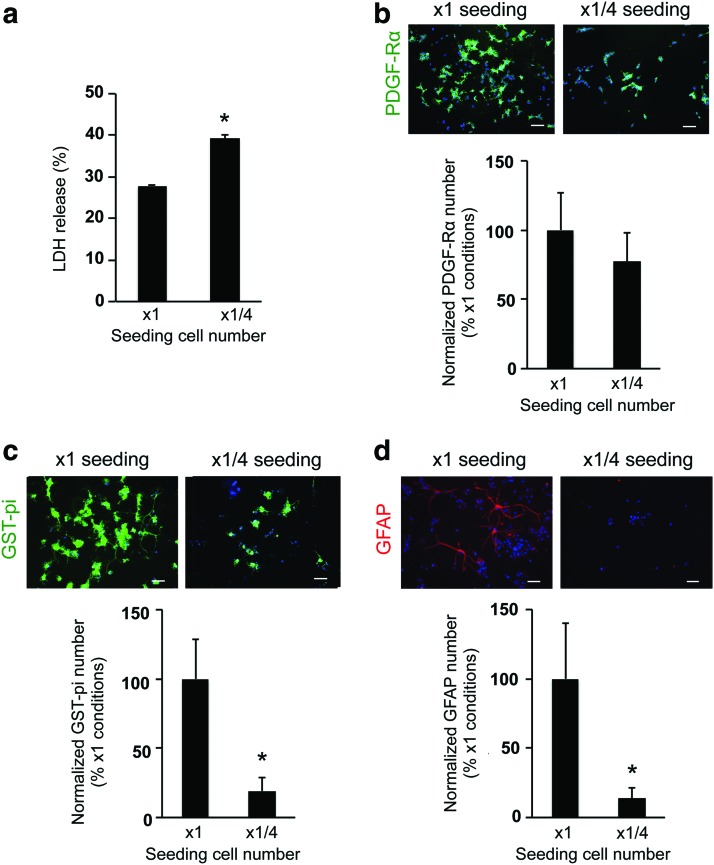
We also compared OPC function between the control seeding cell number (3,600 cells/well in 1 mL) and the lower seeding cell number (900 cells/well in 1 mL) conditions. On day 3, the wells with lower seeding cell number (e.g., 1/4× seeding cell number) had higher values of LDH release compared to the control condition (e.g., 1× seeding cell number) (Fig. 5a). The lower seeding cell number was associated with a slightly lower OPC number during the first 3 days after cell seeding in the gel (Fig. 5b; but there was no significant difference between the two groups). On the contrary, OPC differentiation into oligodendrocytes (Fig. 5c) and minor conversion into astrocytes (Fig. 5d) were robustly and significantly suppressed with a lower seeding cell number. However, even with lower seeding cell number, reducing the gel concentration and viscosity promoted the cell survival and differentiation (Supplementary Fig. S3), supporting the idea that the gel concentration and cell-seeding density are both critical factors for OPC survival and differentiation in our 3D culture system.
FIG. 5.
Comparative design: high-density or low-density conditions in hydrogel. (a) Lower numbers of cell-seeding conditions showed higher percentage of LDH release on day 3. *P < 0.01 of N = 4, mean ± SD. (b) PDGF-Rα staining on day 3. The lower numbers of cell-seeding conditions did not affect the OPC proliferation over the first 3 days after cell seeding. N = 6, mean ± SD. Scale bar: 100 μm. (c) GST-pi staining on day 10. The lower numbers of cell-seeding conditions suppressed the differentiation of OPCs into oligodendrocytes. *P < 0.01 of N = 6, mean ± SD. Scale bar: 100 μm. (d) GFAP staining at day 10. The lower numbers of cell-seeding conditions also suppressed the minor conversion of OPCs to astrocytes. *P < 0.01 of N = 5, mean ± SD. Scale bar: 100 μm. Color images available online at www.liebertpub.com/scd
Notch signaling is involved in OPC function in 3D culture system
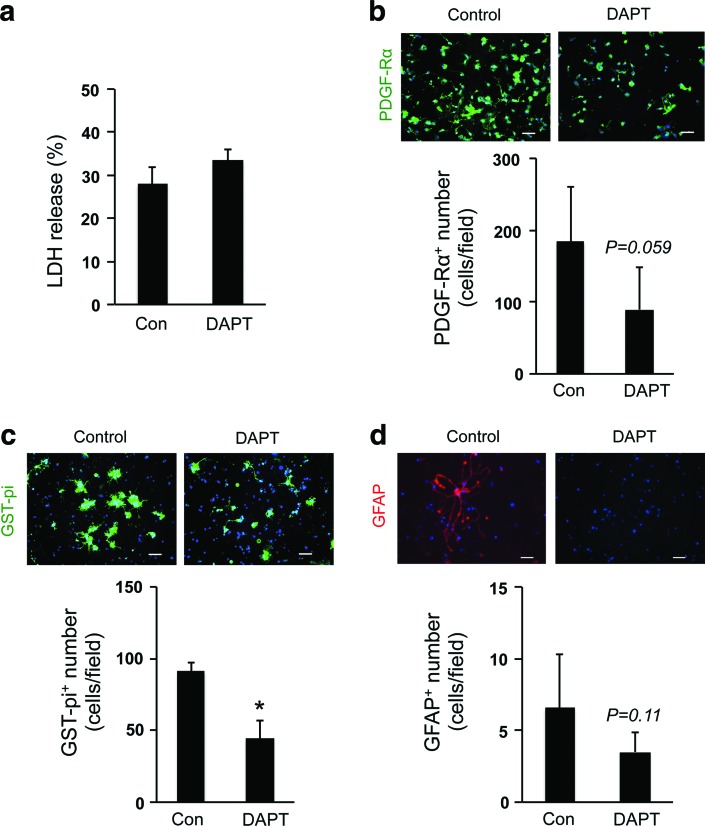
Finally, we examined the role of Notch signaling in our 3D OPC culture system since Notch-mediated signaling supports cell-to-cell communication, which coordinates cell fate during brain development [14,15]. We inhibited Notch signaling in OPCs by adding the well-validated Notch inhibitor DAPT [16]. DAPT did not change the cell viability of OPCs (Fig. 6a), but decreased the number of OPCs on day 3 (Fig. 6b) and oligodendrocytes on day 10 (Fig. 6c), and slightly suppressed the minor conversion into astrocytes (Fig. 6d). Taken together, these data may suggest that cell–cell interaction in the gel is an important mechanism for promoting OPC proliferation and differentiation.
FIG. 6.
Notch signaling in OPCs: (a) a Notch inhibitor DAPT (1 μM) did not induce overt cell death in OPCs in the 3D culture. N = 4, mean ± SD. (b–d) However, DAPT treatment reduced the OPC proliferation during the first 3 days after cell seeding (b), the OPC differentiation into oligodendrocyte between day 3 and 10 (c), and the minor conversion of OPCs to astrocytes (d). *P < 0.01 of N = 5–6, mean ± SD. Scale bar: 100 μm. Color images available online at www.liebertpub.com/scd
Discussion
OPCs are important cells in generating oligodendrocytes during development. Furthermore, even in adult brain, OPCs are widely distributed, comprising ∼5% of all brain cells [17–19]. OPCs in adult brain are quiescent, but after brain damage, those residual OPCs rapidly proliferate and differentiate into mature oligodendrocytes to restore myelin sheaths [20–22]. Although the processes for OPC-to-oligodendrocyte differentiation are relatively well defined in cell culture models [1], intracellular or intercellular mechanisms, which regulate OPC differentiation, still remain to be elucidated. One potential issue is that most in vitro studies assessing OPC function have used 2D culture systems, which may not be able to model the cellular interactions that occur in three dimensions in the brain. In this study, we developed a novel 3D culture system, wherein OPCs proliferated and differentiated into mature oligodendrocytes, enabling the study of OPC function in 3D conditions. As far as we know, there was no report that describes OPC cultures in a 3D condition, except for a related report by Mei et al. [23]. The study described the use of a screening platform with a micropillar system for assessing myelination by preparing OPC cultures on a nonbiomaterial pseudo-axonal substrate. On the micropillars, OPCs survived and differentiated into oligodendrocytes, while wrapping around the micropillars in a 3D manner. This study provides additional new insights into the research field of 3D OPC culture systems; we maintained OPC cultures in biomaterial-based gels and the cells successfully proliferated and differentiated in the gel. Our system maintains OPC cultures in biomaterial-based gels, which allow OPC access to neighboring cells in all (360°) directions. Therefore, our 3D OPC culture system offers a unique approach for examining the regulatory mechanisms for OPC function in conditions that more closely approximate the in vivo situation than 2D culture systems.
Another important finding of this study is that the OPC-to-astrocyte conversion in our 3D OPC culture system mirrors that observed in vivo. Historically, OPCs from rat optic nerves have been identified as bipotential oligodendrocyte–type-2 astrocyte progenitor cells (so called O-2A cells) that differentiate not only into oligodendrocytes but also into astrocytes [24]. Studies of fate-mapping analysis of OPCs using NG2-creER transgenic mice showed that NG2-positive OPCs undergo robust conversion into astrocytes prenatally, a process that ceases shortly after birth [12,13]. Other studies indicate that cell–cell contact may regulate OPC cell division and the ability to differentiate along restricted lineages during development [1]. Because the regulatory mechanisms of cell fate of OPCs are still mostly unknown, our new 3D OPC culture system can be a new tool to investigate the mechanisms of OPC differentiation in vitro.
Our study may also contain one important clinical implication for cell-based therapy. Stem cell therapy is now expected as an effective approach for several central nervous system (CNS) diseases, including stroke [25–27]. Also importantly, OPCs are now proposed as a new source for cell-based therapy. In fact, recent preclinical studies reported that transplanted OPCs could improve remyelination in animal models of demyelination [28–32]. These studies prepared OPCs from immature stem cells (e.g., embryonic stem cells, neural stem cells, or induced pluripotent stem cells) in vitro and then transplanted the cells after collecting them in liquid. Importantly, the collagen/hyaluronan gels that we used in this study can be transplanted to rodent/human bodies. Therefore, our 3D OPC cultures may be another approach to prepare OPCs for cell transplantation in CNS disease patients.
Taken together, we have developed a novel 3D OPC culture system, wherein seeded OPCs can proliferate and differentiate into mature oligodendrocytes. Nevertheless, there are some important caveats and limitations that need to be carefully assessed in future studies. First, we did not investigate why increasing gel concentration (e.g., gel viscosity) and reducing seeding cell number suppressed OPC proliferation and differentiation in our 3D culture system. These changes may decrease the accessibility to neighboring cells in the gels because (i) increases in gel concentration/viscosity would reduce the cell motility in gels and (ii) reducing seeding cell number would lower the chance for seeding cells to interact with neighboring cells, which both may impede cell–cell interaction in the culture conditions. In general, cell–cell interaction is critical for maintaining proper cellular function, including cell proliferation and differentiation [33–35]. Indeed, this study demonstrated that Notch signaling plays important roles in supporting OPC function in our 3D system. Therefore, in future studies, it would be needed to investigate how gel concentration and/or seeding cell density affect the cell–cell interaction in the 3D OPC culture system. Second, we only focused on the role of Notch signaling as a mechanism for cell–cell contact in our 3D OPC cultures. However, other cell surface proteins and secreted factors also mediate trophic coupling between cells in the 3D gel. To understand the mechanisms of cell–cell interactions in OPC cultures more thoroughly, we need to carefully dissect the factors that mediate intercellular interactions. Third, this study assessed the number of OPCs/oligodendrocytes to investigate the function of OPC proliferation/differentiation in our 3D culture system. However, our 3D system will require further investigations on the cell functionality and maturation, or cell–cell interaction, to fully appreciate the applications of our model when characterizing cell behavior and fate. Therefore, in future studies, more assays of OPC function (e.g., BrdU labeling to assess OPC proliferation/differentiation) and oligodendrocyte function (e.g., myelin synthesis) are warranted to dissect the mechanisms of oligodendrocyte lineage cells. Fourth, we assumed that increases in gel viscosity (by increasing the ratio of hyaluronan to media) suppressed OPC proliferation and differentiation by physically suppressing OPC movement toward neighboring OPCs. However, hyaluronan could chemically inhibit OPC maturation through Toll-like receptor 2 pathways under some conditions [36]. Therefore, understanding how gel components chemically affect OPC function would be important for improving our newly developed 3D culture system. Fifth, although cell cultures in 3D system may display different characteristics in their cellular function compared to the ones in 2D system [37], this study did not directly compare OPC function in 3D versus 2D culture conditions. Our preliminary experiment suggests that some differences may exist in DAPT-mediated effects for OPC maturation between 2D and 3D conditions (data not shown). Therefore, the wide application of our new culture system will require a careful comparison with conventional ones to further reveal the advantages and disadvantages of utilizing 2D and 3D culture paradigms. And finally, we cultured OPCs in collagen and hyaluronan gels because collagen/hyaluronan gels can be used as a material for cell-based therapies, such as stem cell transplantation, which is now expected to be a potentially effective approach for several CNS diseases. Therefore, in future studies, it may be worthwhile testing whether a coculture system of OPCs with other brain cells, such as neurons, can be developed in the 3D collagen and hyaluronan gel.
In summary, we report on the successful development of a novel 3D culture system for OPCs. In the 3D gels, OPCs proliferate and differentiate into oligodendrocytes with a minor conversion into astrocytes, which mirrors the phenomena in vivo. This 3D OPC culture system provides a new in vitro approach for the analysis of OPC function.
Supplementary Material
Acknowledgment
The study was supported, in part, by National Institutes of Health, Mitsui Sumitomo Insurance Welfare Foundation, and Uehara Memorial Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Nishiyama A, Komitova M, Suzuki R. and Zhu X. (2009). Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci 10:9–22 [DOI] [PubMed] [Google Scholar]
- 2.Gamba A, Ambrosi D, Coniglio A, de Candia A, Di Talia S, Giraudo E, Serini G, Preziosi L. and Bussolino F. (2003). Percolation, morphogenesis, and burgers dynamics in blood vessels formation. Phys Rev Lett 90:118101. [DOI] [PubMed] [Google Scholar]
- 3.Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D'Avanzo C, Chen H, Hooli B, Asselin C, et al. (2014). A three-dimensional human neural cell culture model of Alzheimer's disease. Nature 515:274–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Itoh K, Maki T, Shindo A, Egawa N, Liang AC, Itoh N, Lo EH, Lok J. and Arai K. (2016). Magnesium sulfate protects oligodendrocyte lineage cells in a rat cell-culture model of hypoxic-ischemic injury. Neurosci Res 106:66–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyamoto N, Maki T, Shindo A, Liang AC, Maeda M, Egawa N, Itoh K, Lo EK, Lok J, Ihara M. and Arai K. (2015). Astrocytes promote oligodendrogenesis after white matter damage via brain-derived neurotrophic factor. J Neurosci 35:14002–14008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piao JH, Wang Y. and Duncan ID. (2013). CD44 is required for the migration of transplanted oligodendrocyte progenitor cells to focal inflammatory demyelinating lesions in the spinal cord. Glia 61:361–367 [DOI] [PubMed] [Google Scholar]
- 7.Davis GE. (1992). Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun 182:1025–1031 [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Ceylan M, Shrestha B, Wang H, Lu QR, Asmatulu R. and Yao L. (2014). Nanofibers support oligodendrocyte precursor cell growth and function as a neuron-free model for myelination study. Biomacromolecules 15:319–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang DG, Tokumoto YM. and Raff MC. (2000). Long-term culture of purified postnatal oligodendrocyte precursor cells. Evidence for an intrinsic maturation program that plays out over months. J Cell Biol 148:971–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arai K. and Lo EH. (2009). An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci 29:4351–4355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arai K. and Lo EH. (2010). Astrocytes protect oligodendrocyte precursor cells via MEK/ERK and PI3K/Akt signaling. J Neurosci Res 88:758–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang W, Zhao N, Bai X, Karram K, Trotter J, Goebbels S, Scheller A. and Kirchhoff F. (2014). Novel NG2-CreERT2 knock-in mice demonstrate heterogeneous differentiation potential of NG2 glia during development. Glia 62:896–913 [DOI] [PubMed] [Google Scholar]
- 13.Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R. and Nishiyama A. (2011). Age-dependent fate and lineage restriction of single NG2 cells. Development 138:745–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y. and Cheng R. (2006). Viscometric study of gelatin in dilute aqueous solutions. J Polym Sci B Polym Phys 44:1804–1812 [Google Scholar]
- 15.Ables JL, Breunig JJ, Eisch AJ. and Rakic P. (2011). Not(ch) just development: notch signalling in the adult brain. Nat Rev Neurosci 12:269–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Wang J, Zhao C, Ren K, Xia Z, Yu H. and Jiang K. (2016). Acute blockage of Notch signaling by DAPT induces neuroprotection and neurogenesis in the neonatal rat brain after stroke. Transl Stroke Res 7:132–140 [DOI] [PubMed] [Google Scholar]
- 17.Levine JM, Reynolds R. and Fawcett JW. (2001). The oligodendrocyte precursor cell in health and disease. Trends Neurosci 24:39–47 [DOI] [PubMed] [Google Scholar]
- 18.Pringle NP, Mudhar HS, Collarini EJ. and Richardson WD. (1992). PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development 115:535–551 [DOI] [PubMed] [Google Scholar]
- 19.Dawson MR, Polito A, Levine JM. and Reynolds R. (2003). NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24:476–488 [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto N, Tanaka R, Shimura H, Watanabe T, Mori H, Onodera M, Mochizuki H, Hattori N. and Urabe T. (2010). Phosphodiesterase III inhibition promotes differentiation and survival of oligodendrocyte progenitors and enhances regeneration of ischemic white matter lesions in the adult mammalian brain. J Cereb Blood Flow Metab 30:299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skihar V, Silva C, Chojnacki A, Doring A, Stallcup WB, Weiss S. and Yong VW. (2009). Promoting oligodendrogenesis and myelin repair using the multiple sclerosis medication glatiramer acetate. Proc Natl Acad Sci U S A 106:17992–17997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gensert JM. and Goldman JE. (1997). Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron 19:197–203 [DOI] [PubMed] [Google Scholar]
- 23.Mei F, Fancy SP, Shen YA, Niu J, Zhao C, Presley B, Miao E, Lee S, Mayoral SR, et al. (2014). Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat Med 20:954–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raff MC, Miller RH. and Noble M. (1983). A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 303:390–396 [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Frutos B, Otero-Ortega L, Gutierrez-Fernandez M, Fuentes B, Ramos-Cejudo J. and Diez-Tejedor E. (2016). Stem cell therapy and administration routes after stroke. Transl Stroke Res 7:378–387 [DOI] [PubMed] [Google Scholar]
- 26.Napoli E. and Borlongan CV. (2016). Recent advances in stem cell-based therapeutics for stroke. Transl Stroke Res 7:452–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oppliger B, Joerger-Messerli M, Mueller M, Reinhart U, Schneider P, Surbek DV. and Schoeberlein A. (2016). Intranasal delivery of umbilical cord-derived mesenchymal stem cells preserves myelination in perinatal brain damage. Stem Cells Dev 25:1234–1242 [DOI] [PubMed] [Google Scholar]
- 28.Kim JB, Lee H, Arauzo-Bravo MJ, Hwang K, Nam D, Park MR, Zaehres H, Park KI. and Lee SJ. (2015). Oct4-induced oligodendrocyte progenitor cells enhance functional recovery in spinal cord injury model. EMBO J 34:2971–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M. and Goldman SA. (2013). Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 12:252–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharp J, Frame J, Siegenthaler M, Nistor G. and Keirstead HS. (2010). Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells 28:152–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K. and Steward O. (2005). Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci 25:4694–4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G, 2nd, Roy NS. and Goldman SA. (2004). Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat Med 10:93–97 [DOI] [PubMed] [Google Scholar]
- 33.Wei Q, Hariharan V. and Huang H. (2011). Cell-cell contact preserves cell viability via plakoglobin. PLoS One 6:e27064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green KJ, Getsios S, Troyanovsky S. and Godsel LM. (2010). Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb Perspect Biol 2:a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karsenti E. (2008). Self-organization in cell biology: a brief history. Nat Rev Mol Cell Biol 9:255–262 [DOI] [PubMed] [Google Scholar]
- 36.Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B. and Vartanian T. (2010). Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A 107:11555–11560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedl P, Sahai E, Weiss S. and Yamada KM. (2012). New dimensions in cell migration. Nat Rev Mol Cell Biol 13:743–747 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.