Abstract
In untreated HIV infection, CD8+ T cell exhaustion (i.e., decreased proliferative and effector capacity) is associated with high levels of expression of coinhibitory receptors, including PD-1, T cell immunoreceptor with Ig and ITIM domains (TIGIT), CD160, and 2B4. This is evident for both HIV-specific and non-HIV-specific CD8+ T cells. Antiretroviral therapy (ART) initiated during chronic infection decreases but may not completely normalize the expression of such “exhaustion markers.” Compared to initiation of ART later in the course of disease, initiation soon after infection reduces some parameters of chronic inflammation and adaptive immune dysfunction. However, it is not known if Early ART (e.g., initiated within the first 6 months after HIV infection) versus Delayed ART (e.g., initiated during chronic infection) preferentially reduces expression of exhaustion markers. We evaluated exhaustion marker expression on subsets of circulating effector and memory CD8+ T cells at longitudinal pre- and post-ART (2 and 5 years on ART) time points from n = 19 (Early ART) and n = 23 (Delayed ART) individuals. Before ART, TIGIT and CD160 were expressed on a statistically significantly higher proportion of effector and transitional memory cells from individuals in the Delayed ART group: the timing of ART initiation, however, did not consistently affect the expression of the exhaustion markers once viral suppression was achieved. Understanding which factors do and do not regulate aspects of CD8+ T cell exhaustion, including the expression of exhaustion markers, is critical to inform the rational design of CD8+ T cell-based therapies to treat HIV, for which CD8+ T cell exhaustion remains an important barrier to efficacy.
Keywords: : HIV, CD8+ T cell, exhaustion, Early ART
Introduction
Chronic untreated HIV disease is associated with high levels of inflammation and expression of markers of activation and exhaustion on T cells.1–3 Although durable viral suppression with antiretroviral therapy (ART) significantly reduces inflammation and immune activation, some markers of immune activation and T cell exhaustion remain elevated at a level above that seen in uninfected individuals.4–8 T cell exhaustion may plausibly contribute to increased morbidity and mortality in HIV-infected individuals on ART, and therefore, it is important to understand how this state is regulated.
In recent years, the expression of several inhibitory receptors on the surface of T cells has been described in HIV infection, as well as in other settings of persistent antigenic and inflammatory signals, such as other chronic infections and tumors. These inhibitory receptors, which include PD-1, T cell immunoreceptor with Ig and ITIM domains (TIGIT), CD160, and 2B4, are highly expressed on CD4+ and CD8+ T cells during prolonged exposure to antigen and some inflammatory cytokines.8–12 High inhibitory receptor expression and coexpression are associated with functionally exhausted CD8+ T cells, which have reduced effector functions and proliferative capacity.2,12 In untreated HIV infection, high expression of these exhaustion markers is also observed on non-HIV-specific bulk CD8+ T cells.2,11 As expression of inhibitory receptors correlates with CD8+ T cell function, it is critical to understand how ART and, in particular, the timing of ART impact their expression on both bulk and HIV-specific CD8+ T cells.
Initiating ART soon after HIV is acquired limits chronic inflammation and T cell activation and may decrease the size of the HIV reservoir.6,13–16 In this study, we asked whether early initiation of ART is also associated with a lower level of exhaustion marker expression on bulk CD8+ T cells. This question has been evaluated previously by our group for PD-1, the expression of which is not preferentially reduced by Early ART.17 Other groups have also determined that the expression of PD-1, 2B4, and CD160 is not lower in bulk CD8+ T cells from individuals treated with ART at a higher CD4+ T cell count versus those who initiated therapy at a lower CD4+ T cell count (both during the chronic phase of infection).18 However, it remains unknown whether initiating ART during the early months after HIV infection reduces the expression and/or coexpression of these additional markers. To address this question, we evaluated the expression of the exhaustion markers PD-1, TIGIT, CD160, and 2B4 on circulating effector and memory CD8+ T cell subsets in longitudinal peripheral blood samples from individuals treated early in infection (e.g., within the first 6 months after acquisition) versus individuals treated during chronic, established infection (e.g., at least 18 months after the date of infection).
Materials and Methods
Study subjects and samples
This study sampled HIV-infected participants (and an HIV-uninfected control) from the Zuckerberg San Francisco General Hospital clinic-based SCOPE and Options Project cohorts. The UCSF Committee on Human Research approved this study, and participants provided informed, written consent before enrollment. The SCOPE cohort enrolls chronically HIV-infected individuals with an unknown HIV infection date. The Options Project enrolls individuals <12 months after HIV antibody seroconversion (after 2003, this was restricted to <6 months after seroconversion). Dates of HIV infection for Options participants were defined as the first date on which HIV would likely have been detected in a given patient, using a sensitive RNA assay. This “estimated date of detectable infection” was inferred from the detailed history of HIV test seroconversion for each individual, and calculated using a previously described method19 from the known conversion delays for different HIV diagnostic tests.20,21
For this study, we selected SCOPE or Options cohort participants who were enrolled between 1996 and 2013, who had a documented positive HIV antibody test, and who were not on ART at the time of enrollment. Individuals were classified into one of two groups: (1) Early ART group (started on ART during early HIV infection): participants from Options who had a documented pre-ART HIV viral load (VL) >5,000 copies/ml, started ART <6 months after estimated date of HIV infection, or (2) Delayed ART group (started on ART during chronic HIV infection): participants were selected either (i) from Options if they started ART >18 months after estimated HIV infection date or (ii) from SCOPE, where precise date of HIV infection is unknown but confirmed to be >18 months before the start of ART. Both groups achieved a plasma HIV RNA level <75 copies/ml by month 6 of ART and continuously maintained viral suppression throughout the sampling period for up to 5 years. Peripheral blood mononuclear cell (PBMC) samples were selected, as available, at the following three time points: pre-ART [as close to the time of starting ART as possible given sample availability, 2 years on ART (±6 months), and 5 years on ART (±6 months)]. Observations following initial viral suppression were excluded after any documented VL measurement >2,000 copies/ml (no included participants had VL >300 copies/ml measured after suppression was achieved). CD4+ T cell counts and HIV-1 plasma RNA levels were measured in all cohort participants by CLIA-certified clinical laboratory assays at the time of entry into the cohort and approximately every 3–4 months thereafter.
Phenotyping by flow cytometry
Cryopreserved PBMCs were thawed and 2–4 million cells were stained for 20 min on ice with fixable viability dye to allow for discrimination of dead cells (eFluor 506; Affymetrix, Santa Clara, CA). Cells were stained in FACS buffer (phosphate-buffered saline [PBS] plus 2% fetal bovine serum) at room temperature for 20 min with antibodies to detect expression of surface markers. Antibodies were obtained from BioLegend (San Diego, CA: anti-CD14 and anti-CD19−BV510− clones M5E2, HIB19− used as dump gates, anti-PD-1 BV421 or BV605 clone EH12.2H7, anti-CD27 BV570 clone O323, anti-CD4 BV711 clone OKT4, anti-CCR7 BV785 clone G043H7, anti-2B4 FITC clone C1.7, anti-CD160 PE-Cy7 clone BY55, anti-CD45RA APC-Cy7 clone H100), BD Biosciences (San Jose, CA: anti-CD3 BV650 clone SK7, anti-CD8α APC-R700 clone RPA-T8; BD), and Affymetrix (anti-TIGIT APC clone MBSA43). After staining, cells were fixed with 0.5% paraformaldehyde in PBS and immediately analyzed on an LSRII flow cytometer (BD). Cytometer settings were standardized between experiments using Application settings. PBMCs from a single HIV-uninfected individual (processed and cryopreserved on the same day) were run in parallel in all experiments. Flow cytometry panel was optimized using fluorescence-minus-one controls, and gating for the exhaustion markers was standardized between experiments by fixing the gate according to the proportion of effector memory CD8+ T cells (Tem: CD45RA−CCR7−CD27−) from the control donor that expressed the exhaustion marker. To further adjust for experiment-to-experiment variation, all experiments were performed within a 30-day period. In addition, longitudinal samples from each individual study participant were thawed, stained, and run on the flow cytometer on the same experiment day. Data were analyzed using FlowJo v10.1 software (Tree Star, Ashland, OR).
Statistical analysis
Comparisons of CD4+ T cell count nadir and pre-ART VL between the Early and Delayed ART groups were performed using the Wilcoxon rank sum test. All parameters measured by flow cytometry were compared between patients with Early ART start and Delayed ART start at each corresponding time point using t-tests. To assess potential changes within individuals, paired t-tests were used to compare measurements at each on-ART time to the pre-ART time within each patient group. To estimate associations between parameters measured by flow cytometry and clinical parameters (e.g., concurrent VL or infection duration at ART initiation), linear regression models were run separately for each patient group and time point with the flow cytometry measure as the outcome and the clinical parameter as a single predictor. Analyses were run using Stata v14 (Stata Corp., College Station, TX).
Results
Study population
Clinical characteristics of the subjects in the Early and Delayed ART groups are summarized in Table 1. The majority of participants were male (100% in the Early group, 94% in the Delayed group) and the median age of participants at the initiation of ART was similar between the groups (44 and 43 years, respectively), as were the ART regimens. As expected, the Early ART group had a higher median pre-ART nadir CD4+ T cell count than the Delayed ART group (538 cells/mm3 vs. 377 cells/mm3, p = .04), and a higher median pre-ART plasma HIV RNA level (133,400 copies/ml vs. 64,616 copies/ml, p = .02). The Early ART group continued to have higher median CD4+ T cell counts than the Delayed ART group at 2 years (883 cells/mm3 vs. 528 cells/mm3, p < .01) and at 5 years of ART-mediated viral suppression (809 cells/mm3 vs. 665 cells/mm3, p = .03).
Table 1.
Summary of Participant Clinical Characteristics
| CD4 T cells (cells/mm3) | |||||||
|---|---|---|---|---|---|---|---|
| Age, years (ART start) | Sex | Days between EDI and ART start | VL pre-ART (cells/ml) | Pre-ART | 2 Years ART | 5 Years ART | |
| Early ART | 37 | Male | 75 | 7,988 | 538 | 673 | 623 |
| 45 | Male | 156 | 289,235 | 369 | 571 | — | |
| 54 | Male | 49 | 103,595 | 846 | 987 | 1,404 | |
| 34 | Male | 176 | 1,244,229 | 551 | 695 | 847 | |
| 40 | Male | 44 | 11,066 | 595 | 915 | — | |
| 27 | Male | 85 | 35,326 | — | 1,029 | — | |
| 34 | Male | 65 | 133,400 | 445 | — | 832 | |
| 47 | Male | 49 | 75,068 | 509 | 692 | 744 | |
| 25 | Male | 67 | 62,761 | 549 | 883 | 908 | |
| 53 | Male | 28 | 2,527,663 | 521 | 714 | 893 | |
| 52 | Male | 21 | 750,000 | — | 910 | — | |
| 54 | Male | 49 | 500,000 | — | 1,209 | 856 | |
| 45 | Male | 23 | 500,000 | 190 | — | 704 | |
| 50 | Male | 182 | 148,840 | 583 | — | 619 | |
| 22 | Male | 60 | 55,557 | 875 | — | 701 | |
| 41 | Male | 84 | 114,843 | — | 951 | 786 | |
| 48 | Male | 124 | 128,006 | 449 | 498 | — | |
| 40 | Male | 29 | 237,725 | 730 | 1,365 | — | |
| 44 | Male | 28 | 586,073 | 136 | 394 | — | |
| Median | 44 | — | 60 | 133,400 | 538 | 883 | 809 |
| Delayed ART | 43 | Male | 5,177 | 10,732 | 377 | 607 | — |
| 53 | Female | 2,181 | 28,896 | 214 | 570 | 421 | |
| 50 | Male | 814 | 49,330 | 295 | 460 | 755 | |
| 42 | Male | — | 92,400 | 249 | 338 | — | |
| 41 | Male | — | 9,211 | 336 | 407 | — | |
| 39 | Male | 1,692 | 4,774 | 441 | 529 | 665 | |
| 32 | Male | 615 | 66,018 | 610 | 896 | — | |
| 47 | Male | 631 | 82,713 | — | 526 | — | |
| 47 | Male | — | 54,100 | 886 | 1,020 | — | |
| 39 | Male | 2,124 | 22,907 | 462 | 444 | 498 | |
| 27 | Male | 942 | 271,630 | — | 583 | 452 | |
| 40 | Male | — | 132,724 | — | 526 | — | |
| 27 | Male | — | 71,281 | 348 | 619 | — | |
| 35 | Male | — | 150,167 | — | — | 535 | |
| 56 | Male | 945 | 356,000 | — | 411 | 517 | |
| 37 | Male | 897 | 64,616 | 435 | 502 | 740 | |
| 52 | Male | — | 34,031 | 381 | 702 | — | |
| 52 | Male | 1,207 | 286,420 | 265 | 862 | 778 | |
| 43 | Male | 928 | 29,762 | 331 | 650 | — | |
| 52 | Male | 554 | 21,233 | 528 | 659 | 804 | |
| 62 | Male | 729 | 64,411 | 304 | 524 | — | |
| 52 | Male | 1,459 | 162,600 | — | 398 | — | |
| 39 | Male | 674 | 66,552 | 471 | 445 | 855 | |
| Median | 43 | — | 935 | 64,616 | 377 | 528 | 665 |
Clinical characteristics of individual study participants (and median values) in the Early and Delayed ART groups.
ART, antiretroviral therapy; EDI, estimated date of infection; VL, viral load.
Distribution of CD8+ T cell subsets is statistically significantly different between early versus chronic untreated HIV infection
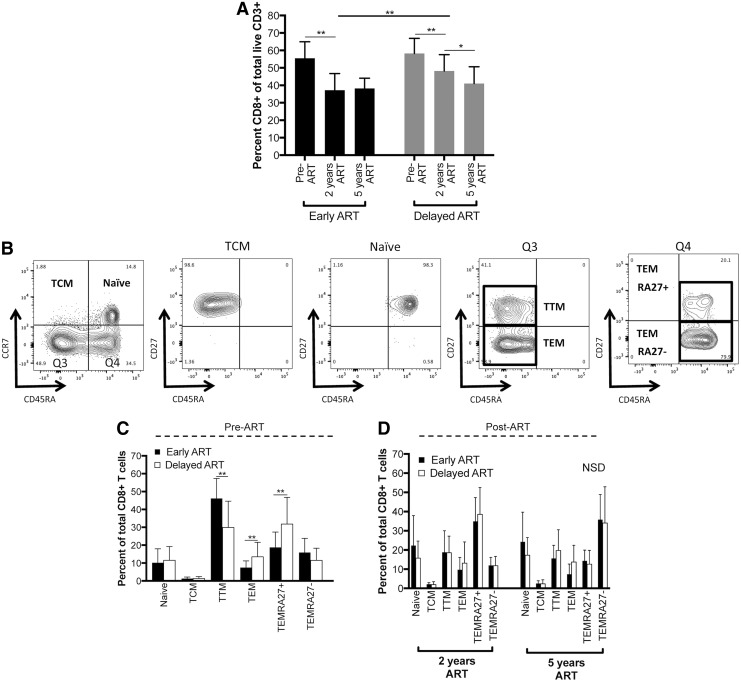
We first explored the distribution of CD8+ T cell subsets in the Early versus Delayed ART groups. The overall proportion of CD3+ T cells that was CD8+ in the peripheral blood declined after the initiation of ART, likely corresponding to CD4+ T cell count recovery (Fig. 1A and Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/aid). In both the Early and Delayed ART groups, there was a statistically significant decline in the percent of CD8+ T cells between the pre-ART and 2 years post-ART time points. The Delayed ART group also demonstrated a further decline in the percent of CD8+ T cells between 2 and 5 years on ART. There was a statistically significant difference in the percent of CD8+ T cells between the Early and Delayed groups after 2 years on ART. This difference between the groups did not persist after 5 years on ART.
FIG. 1.
Peripheral blood CD8+ T cell subset distribution in Early and Delayed ART groups. PBMCs were thawed and stained with fluorescently labeled antibodies for flow cytometric analysis. (A) Early and Delayed ART groups demonstrated a similar overall percent of live CD3+ cells that are CD8+ before the initiation of ART (gating strategies shown in Supplementary Fig. S1). After ART initiation, the percent was significantly higher in the Delayed ART group after 2 years of ART, however, the difference normalized after 5 years of continuous viral suppression on ART. (B) Gating strategy for naive, effector, and memory CD8+ T cell subsets. CD27 expression is shown on all four quadrants (Q) from the initial subsets defined by CD45RA and CCR7 expression (leftmost plot). Definition of subsets: naive (CD45RA+CCR7+), TCM (central memory; CD45RA−CCR7+), TTM (transitional memory; CD45RA−CCR7−CD27+), TEM (effector memory; CD45RA−CCR7−CD27−), TEMRA27+ (CD45RA+CCR7−CD27+), TEMRA27− (CD45RA+CCR7−CD27−). (C, D) Summary of the percentage of each CD8+ T cell effector-memory subset in the two treatment groups at three time points [pre-ART (C), after 2 and 5 years on ART (D)]. Only significant differences between the Early and Delayed ART groups within the same subset at the same time point are highlighted with statistical significance bars (statistically significant differences within group across time points not shown). *p < .05, **p < .01. ART, antiretroviral therapy; NSD, no statistically significant differences; PBMC, peripheral blood mononuclear cell.
We next divided the CD8+ T cell compartment into subsets using markers that distinguish populations with different effector and memory properties (Fig. 1B).22,23 Six subsets of live CD3+CD8+ T cells were defined as follows: naive (TN: CD45RA+CCR7+), central memory (TCM: CD45RA−CCR7+), transitional memory (TTM: CD45RA−CCR7+CD27+), effector memory (TEM: CD45RA−CCR7+CD27−), TEMRA CD27+ (TEMRA27+: CD45RA+CCR7−CD27+), and TEMRA CD27− (TEMRA27−: CD45RA+CCR7−CD27−). The distribution of these subsets in the Early and Delayed ART groups at the different time points is shown in Figure 1C and D. The frequency of all six subsets statistically significantly changed within the Early ART group on initiation of ART. For example, the proportion of naive, TCM, TEM, and TEMRA27+ populations increased, while the proportion of TTM and TEMRA27− populations decreased after ART (p < .05 for all comparisons within each T cell subset between pre-ART and 2 years post-ART time point; significance lines indicating these differences are not shown in Fig. 1C, D). There was no statistically significant difference in the distribution of the subsets in the Delayed ART group between the pre-ART time point and after the initiation of ART. In this cohort, there was no evidence for an effect of the timing of ART initiation (Early vs. Delayed) on the frequency of CD8+ T cell effector-memory subsets once virus was suppressed for 2 or 5 years (Fig. 1D). However, statistically significant differences between the Early and Delayed ART groups were observed for three subsets at the viremic pre-ART time point: the proportion of TTM was lower in the Delayed ART group, while the proportion of TEM and TEMRA27+ subsets was higher (p < .01, Fig. 1C).
TIGIT and CD160 are expressed on a greater proportion of TTM and TEM CD8+ T cells in chronic compared to early untreated HIV infection
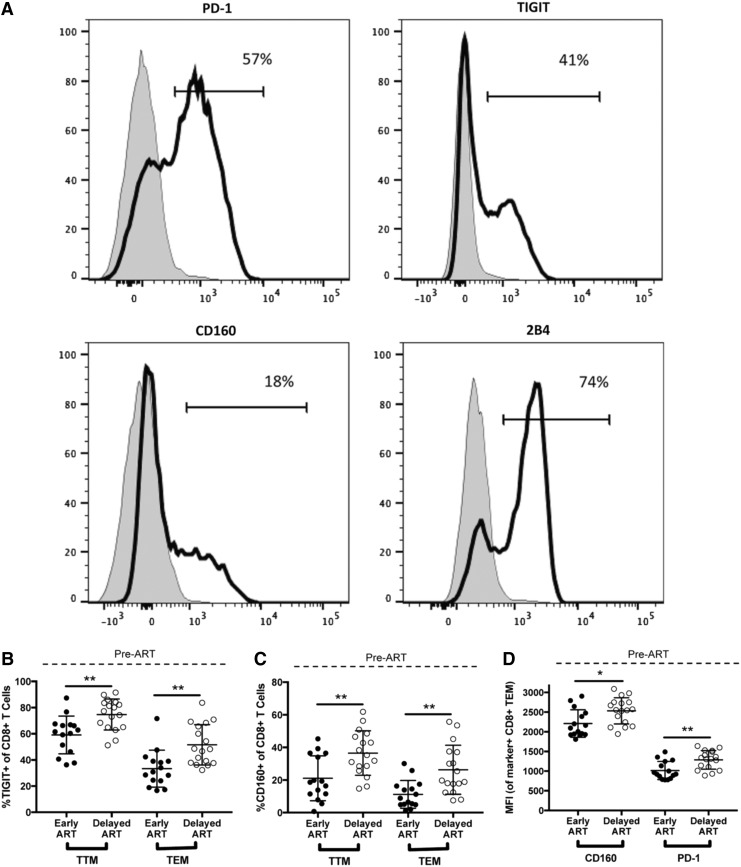
We next characterized the expression of the exhaustion markers PD-1, TIGIT, CD160, and 2B4 on the six CD8+ T cell subsets for the Early and Delayed ART groups before the initiation of therapy (Figs. 2 and 3 and Supplementary Fig. S2). Expression was measured in two ways: the percent of the population that is positive for the marker and the median fluorescence intensity (MFI) of the population that falls within the positive gate, a parameter that reflects the density of protein expression per cell expressing the marker (Fig. 2A). Compared to pre-ART samples from the Delayed ART group, those from the Early ART group exhibited a statistically significantly smaller proportion of TTM and TEM cells that expressed TIGIT and CD160 (Fig. 2B, C; TTM TIGIT 59.1% vs. 74.7%, p < .01; TEM TIGIT 33.4% vs. 51.2%; TTM CD160 21.1% vs. 36.5%, p < .01; TEM CD160 11.2% vs. 26.3%, p < .01). Within the TEM population, the CD160 MFI and the PD-1 MFI were both lower in early compared to chronic infection (Fig. 2C; CD160 MFI 2210 vs. 2531, p = .01; PD-1 MFI 1016 vs. 1286, p < .01). In contrast, we observed an isolated difference in 2B4 expression, with a higher proportion of TCM in the pre-ART samples in the Early ART group expressing 2B4 when compared to the same subset in the pre-ART samples from chronic untreated infection (Fig. 3). The expression of these exhaustion markers at the pre-ART time point did not correlate with concurrent measurements of HIV VL or absolute CD4+ T cell count, nor did it correlate with the number of days between estimated date of HIV infection and ART initiation in the Early ART group (data not shown).
FIG. 2.
Before ART, expression of some exhaustion markers is significantly different in early versus chronic HIV infection. The expression of four T cell exhaustion markers (PD-1, TIGIT, CD160, and 2B4) was evaluated by flow cytometry on the peripheral blood CD8+ T cell subsets described in Figure 1. The level of expression of these markers was determined by both the percent of the subset population that was positive for the marker and the MFI of the marker on the cells that were gated as being positive for the marker. (A) Gating strategy for determining the exhaustion marker-positive populations. Positive gates were drawn in each experiment based on the level of expression of the markers on the TEM CD8+ T cell population from the same HIV-uninfected individual, shown (black line) compared to the same marker expression in the naive CD8+ T cell population from the same individual (gray). Cells from this donor were collected and cryopreserved on the same day, thawed in parallel for all experiments, and used as a consistent gating control. (B–D) Exhaustion markers that are differentially expressed within specific CD8+ T cell subsets in the Early (filled circles) versus Delayed (open circles) ART treatment groups at the pre-ART time point. *p < .05, **p < .01. MFI, median fluorescence intensity; TIGIT, T cell immunoreceptor with Ig and ITIM domains.
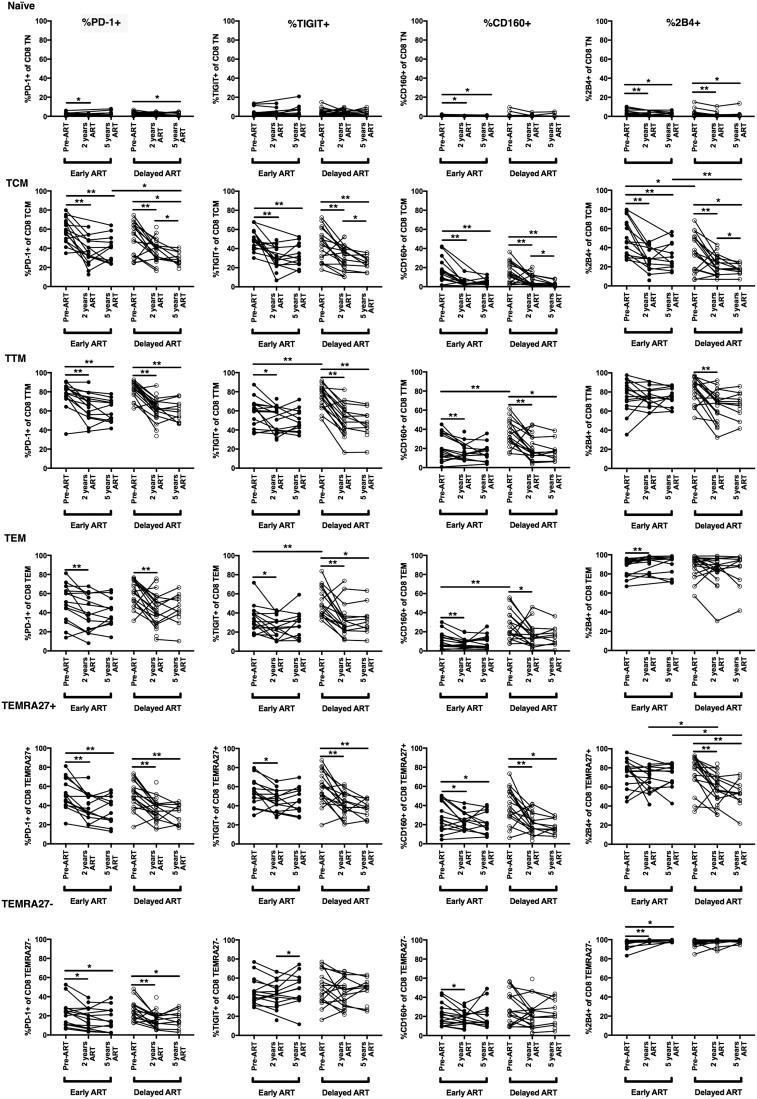
FIG. 3.
Changes in the proportion of CD8+ T cells expressing exhaustion markers across all CD8+ T cell subsets. The expression of four T cell exhaustion markers (PD-1, TIGIT, CD160, and 2B4) was evaluated by flow cytometry on peripheral blood CD8+ T cell subsets, as described in Figure 1. The proportion of cells that are positive for a given marker (%marker+) is shown. *p < .05, **p < .01.
The timing of ART does not systematically impact the expression of most CD8+ T cell exhaustion markers on circulating CD8+ T cell subsets
Across all of the non-naive CD8+ T cell subsets in both treatment groups, the expression of the four exhaustion markers (as measured by percent positive and MFI) decreased between the pre-ART and 2 years post-ART time points to a similar degree in both treatment groups (Fig. 3 and Supplementary Fig. S2). Despite the pre-ART differences discussed above in the proportion of TCM and TTM cells expressing TIGIT and CD160 between the two treatment groups, after therapy was started, the expression of these and most other exhaustion markers in the various CD8+ T cell subsets did not statistically significantly differ depending on the timing of ART initiation, although there were some exceptions. At some but not all post-ART time points, there were potential differences between the treatment groups in isolated markers within isolated subsets (Fig. 3 and Supplementary Fig. S2). Nevertheless, it should be stressed that these potential differences between groups were not consistent across time points and likely would not have remained statistically significant after adjustment for multiple hypothesis testing. Notably, no consistent differences were observed in marker expression between the Early and Delayed ART groups even after excluding the four participants from the Early ART group who were treated between 3 and 6 months after date of infection.
Longer duration of ART impacts the expression of exhaustion markers in some CD8+ T cell subsets only in individuals who initiate ART during chronic infection
Longitudinal sampling allowed us to carefully assess whether the duration of ART affects the expression of exhaustion markers on peripheral CD8+ T cell subsets. Previous studies have demonstrated that HIV DNA reservoirs as well as many markers of chronic immune activation and inflammation reach steady-state levels within the first 1 to 2 years after ART initiation and that a longer duration of therapy does not substantially change these measures further.13,24 We found that this is also the case for most of the exhaustion markers that we studied. One exception was within the CD8+ TCM compartment in individuals from the Delayed ART group. Within this subset, there was a small but statistically significant decrease in the proportion of cells expressing all four exhaustion markers between the 2- and 5-year time points within the Delayed but not Early ART group (Fig. 3).
Discussion
In treated HIV disease, some markers of T cell exhaustion remain persistently elevated.2,8 Because ART initiated within the first few weeks to months of HIV infection can reduce systemic inflammation, we asked whether timing of ART impacts the expression of immunoregulatory inhibitory receptors on non-HIV-specific CD8+ T cells, in particular four markers that are known to be highly expressed on CD8+ T cells with reduced function.12 We performed a comprehensive analysis of four inhibitory receptors on distinct effector and memory subsets of circulating CD8+ T cells in pre- and post-ART samples from individuals who initiated therapy within the first 6 months of infection versus those who initiated therapy at least one and half years after infection. We found that, although the duration of untreated infection impacted the expression of some markers in some CD8+ T cell effector-memory subsets before ART (most strikingly TIGIT and CD160), consistent differences in expression did not appear to be present between the Early and Delayed treatment groups after ART was initiated.
The differences between the two groups that we observed at the viremic time point before ART are notable. First, we found statistically significant differences in the proportion of the CD8+ T cell subsets before ART initiation in the early phase of HIV infection compared to the chronic phase. Specifically, we observed a skew toward less differentiated subsets (i.e., a greater proportion of transitional memory cells) in early infection as opposed to a greater proportion of effector memory and the CD27-expressing TEMRA population in late infection. Second, a higher proportion of transitional and effector memory CD8+ T cells expressed TIGIT and CD160 in chronic untreated infection versus early infection. This has been reported previously for TIGIT, but not for CD160.8 The consistent differential expression of these markers between the treatment groups across several subsets suggests that longer duration of infection before treatment may “imprint” high expression of TIGIT and CD160 on the CD8+ T cell compartment. A formal test of this possibility would require an analysis of longitudinal viremic time points within the same individuals, and mechanistically would best be evaluated with epigenetic approaches.
Notably, even though there is wide interindividual variation in the expression of all of the exhaustion markers that we tested, the expression of none of them at the viremic time point correlated substantially with concurrent VL in either group. In addition, expression of these exhaustion markers did not correlate substantially with the time between estimated date of infection and sampling dates (duration of infection) within the Early ART group. This lack of correlation suggests that other factors, such as host genetic polymorphisms in inflammatory pathways or the presence of coinfections, may contribute to this variation in exhaustion maker expression at the pre-ART time point. Given the relationship between T cell activation and circulating inflammatory mediators, it would be interesting to assess how and whether exhaustion marker expression correlates with levels of inflammatory cytokines known to be elevated in the setting of chronic HIV infection (e.g., type 1 interferons and interleukin 6).
After ART initiation, a handful of exhaustion markers in isolated subsets—and at isolated time points—appeared to be differentially expressed between Early and Delayed ART groups. Nevertheless, there seemed to be no biologically coherent pattern of differentially expressed markers nor was there consistency between frequency and MFI analyses, increasing the likelihood that these potential differences may have been spurious. This lack of difference was surprising to us because several markers of chronic inflammation and viral reservoir size—two factors that are thought to drive upregulation of these markers—have previously been found to be lower in individuals treated early versus late in infection.6,13–16 In addition, we have previously found that another marker of CD8+ T cell function, CD57, is differentially expressed on CD8+ T cells from individuals treated with Early versus Delayed ART.25 This lack of substantial difference may represent the true biology that the timing of ART does not, in fact, alter inhibitory receptor expression on bulk CD8+ T cell populations. Alternatively, other explanations related to the cohort of individuals we studied may account for the fact that we did not observe a difference. First, our confidence intervals do not exclude small or even moderate differences between groups. Next, the Early ART group may not have been treated early enough in the course of infection to differentially reduce systemic levels of signals that upregulate the exhaustion markers we examined compared to the Delayed ART group.6,16 Finally, the expression of exhaustion makers after ART on mostly non-HIV-specific bulk CD8+ T cells may be influenced by whether or not an individual is also coinfected with another chronic pathogen such as hepatitis B or C, information that was not readily available for all individuals studied.26
Despite these limitations, our data provide important insight into the regulation of exhaustion marker expression in CD8+ T cell subsets at different time points during untreated HIV infection (i.e., early after infection vs. in the chronic phase), and into how marker expression changes longitudinally after Early versus Delayed ART is initiated. The wide interindividual variation in exhaustion marker expression despite similar disease status suggests the possibility that effector-memory CD8+ T cells in HIV-positive individuals with higher exhaustion marker expression on their bulk CD8+ T cells might demonstrate varying effector capacities, something that could be tested in future studies. Although this association has not been found for PD-1 expression,27,28 the other exhaustion markers have not been evaluated in this manner. All of the exhaustion markers we evaluated have been described as inhibitors of antigen-specific CD8+ T cell function.2,12 Therefore, it will ultimately be important to interrogate what signals regulate the expression of these markers as well as the general state of exhaustion in HIV-specific CD8+ T cells in both untreated and treated disease states. These questions are an explicit focus of ongoing HIV cure research, and this study provides important context for understanding how these markers change in the bulk, nonantigen-specific populations.
Supplementary Material
Acknowledgments
We sincerely thank all study participants. This work was supported by the National Institutes of Health: 5R32AI060530-07 (R.L.R), 5U19A1096109-05 (S.G.D., J.M.M.), 5K24AI069994 (S.G.D.), R01HD074511 (C.D.P.), and R01AI110271 (P.W.H.). C.D.D. received support from the Philippines Department of Science and Technology. The SCOPE cohort was supported the UCSF/Gladstone Institute of Virology and Immunology CFAR (P30 AI027763), the UCSF Clinical and Translational Research Institute Clinical Research Center (UL1 RR024131), and the CFAR Network of Integrated Systems (R24 AI067039). Additional support for SCOPE was provided by the Delaney AIDS Research Enterprise (DARE; AI096109) and the amfAR Institute for HIV Cure Research (amfAR 1093t01). Options cohort support was also provided by the Bill and Melinda Gates Foundation (OPP1062806) and the Harvey V. Berneking Living Trust. We are grateful to Pamela Odorizzi for thoughtful manuscript edits.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Deeks SG, Kitchen CMR, Liu L, Guo H, Gascon R, Narváez AB, Hunt P, Martin JN, Kahn JO, Levy J, et al. : Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 2004;104:942–947 [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto T, Price DA, Casazza JP, Ferrari G, Nason M, Chattopadhyay PK, Roederer M, Gostick E, Katsikis PD, Douek DC, et al. : Surface expression patterns of negative regulatory molecules identify determinants of virus-specific CD8+ T-cell exhaustion in HIV infection. Blood 2011;117:4805–4815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulware DR, Hullsiek KH, Puronen CE, Rupert A, Baker JV, French MA, Bohjanen PR, Novak RM, Neaton JD, Sereti I, et al. : Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 2011;203:1637–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.French MA, King MS, Tschampa JM, da Silva BA, Landay AL: Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis 2009;200:1212–1215 [DOI] [PubMed] [Google Scholar]
- 5.Hunt PW, Lee SA, Siedner MJ: Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis 2016;214 Suppl 2:S44–S50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sereti I, Krebs SJ, Phanuphak N, Fletcher JL, Slike B, Pinyakorn S, O'Connell RJ, Rupert A, Chomont N, Valcour V, et al. : Initiation of antiretroviral therapy in early HIV infection reduces but does not abrogate chronic residual inflammation. Clin Infect Dis 2016;ciw683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, Martínez-Maza O, Bream JH: The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015;29:463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chew GM, Fujita T, Webb GM, Burwitz BJ, Wu HL, Reed JS, Hammond KB, Clayton KL, Ishii N, Abdel-Mohsen M, et al. : TIGIT marks exhausted T cells, correlates with disease progression, and serves as a target for immune restoration in HIV and SIV infection. PLoS Pathog 2016;12:e1005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel M-R, Delwart E, Sepulveda H, Balderas RS, et al. : Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 2006;12:1198–1202 [DOI] [PubMed] [Google Scholar]
- 10.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. : PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006;443:350–354 [DOI] [PubMed] [Google Scholar]
- 11.Peretz Y, He Z, Shi Y, Yassine-Diab B, Goulet J-P, Bordi R, Filali-Mouhim A, Loubert J-B, El-Far M, Dupuy FP, et al. : CD160 and PD-1 co-expression on HIV-specific CD8 T cells defines a subset with advanced dysfunction. PLoS Pathog 2012;8:e1002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuchroo VK, Anderson AC, Petrovas C: Coinhibitory receptors and CD8 T cell exhaustion in chronic infections. Curr Opin HIV AIDS 2014;9:439–445 [DOI] [PubMed] [Google Scholar]
- 13.Jain V, Hartogensis W, Bacchetti P, Hunt PW, Hatano H, Sinclair E, Epling L, Lee T-H, Busch MP, McCune JM, et al. : Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis 2013;208:1202–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajasuriar R, Wright E, Lewin SR: Impact of antiretroviral therapy (ART) timing on chronic immune activation/inflammation and end-organ damage. Curr Opin HIV AIDS 2015;10:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krebs SJ, Ananworanich J: Immune activation during acute HIV infection and the impact of early antiretroviral therapy. Curr Opin HIV AIDS 2016;11:163–172 [DOI] [PubMed] [Google Scholar]
- 16.Crowell TA, Fletcher JL, Sereti I, Pinyakorn S, Dewar R, Krebs SJ, Chomchey N, Rerknimitr R, Schuetz A, Michael NL, et al. : Initiation of antiretroviral therapy before detection of colonic infiltration by HIV reduces viral reservoirs, inflammation and immune activation. J Int AIDS Soc 2016;19:21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cockerham LR, Jain V, Sinclair E, Glidden DV, Hartogenesis W, Hatano H, Hunt PW, Martin JN, Pilcher CD, Sekaly R, et al. : Programmed death-1 expression on CD4+ and CD8+ T cells in treated and untreated HIV disease. AIDS 2014;28:1749–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen SS, Fomsgaard A, Larsen TK, Tingstedt JL, Gerstoft J, Kronborg G, Pedersen C, Karlsson I: Initiation of antiretroviral therapy (ART) at different stages of HIV-1 disease is not associated with the proportion of exhausted CD8+ T cells. PLoS One 2015;10:e0139573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilcher CD, Porco TC, Kassanjee R, Facente SN, Grebe E, Masciotra S, Norris P, Garrett P, Busch MP, Owen SM, Welte A: A generalizable method with improved accuracy for estimation of HIV infection duration using clinical HIV testing histories. HIV Diagnostics Conference, Atlanta, GA, March23, 2016 [Google Scholar]
- 20.Masciotra S, McDougal JS, Feldman J, Sprinkle P, Wesolowski L, Owen SM: Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. J Clin Virol 2011;52 Suppl 1:S17–S22 [DOI] [PubMed] [Google Scholar]
- 21.Masciotra S, Luo W, Youngpairoj AS, Kennedy MS, Wells S, Ambrose K, Sprinkle P, Owen SM: Performance of the Alere Determine™ HIV-1/2 Ag/Ab Combo Rapid Test with specimens from HIV-1 seroconverters from the US and HIV-2 infected individuals from Ivory Coast. J Clin Virol 2013;58 Suppl 1:e54–e58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanzavecchia A, Sallusto F: Understanding the generation and function of memory T cell subsets. Curr Opin Immunol 2005;17:326–332 [DOI] [PubMed] [Google Scholar]
- 23.Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, Corthesy P, Devevre E, Speiser DE, Rufer N: Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol 2007;178:4112–4119 [DOI] [PubMed] [Google Scholar]
- 24.Wada NI, Bream JH, Martínez-Maza O, Macatangay B, Galvin SR, Margolick JB, Jacobson LP: Inflammatory biomarkers and mortality risk among HIV-Suppressed Men: A multisite prospective cohort study. Clin Infect Dis 2016;63:984–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SA, Sinclair E, Jain V, Huang Y, Epling L, Van Natta M, Meinert CL, Martin JN, McCune JM, Deeks SG, et al. : Low proportions of CD28- CD8+ T cells expressing CD57 can be reversed by early ART initiation and predict mortality in treated HIV infection. J Infect Dis 2014;210:374–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saha B, Choudhary MC, Sarin SK: Expression of inhibitory markers is increased on effector memory T cells during hepatitis C virus/HIV coinfection as compared to hepatitis C virus or HIV monoinfection. AIDS 2013;27:2191–2200 [DOI] [PubMed] [Google Scholar]
- 27.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, Plants J, Seth A, Wilson CC, Deeks SG, et al. : Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014;210:1248–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, Robinson J, Huang Y, Epling L, Martin JN, et al. : Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014;210:1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.