Abstract
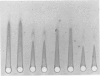
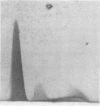
Eighty percent of blood culture isolates of Escherichia coli K-1 are resistant to in vitro opsonophagocytosis by normal human granulocytes and fresh serum. To determine the basis for susceptibility to phagocytosis in 20% of bacteremic K-1 E. coli, we investigated possible quantitative and qualitative immunochemical differences in the K-1 antigen content among resistant and sensitive isolates. We prepared extracts of blood culture K-1 E. coli by sonication and determined the K-1 polysaccharide content per dry weight of bacteria by rocket immunoelectrophoresis using cross-reactive equine anti-group B meningococcal sera. We assessed qualitative differences in the antigen content by crossed immunoelectrophoresis, using an immune globulin fraction and isolated immunoglobulin G (IgG) and IgM from the group B antisera. Three different resistant K-1 isolates contained a mean K-1 content of 48.5 ± 7.6 μg/mg ± standard deviation of dry bacteria, and three sensitive isolates contained 23.2 ± 5.6 μg/mg (P < 0.005). Crossed immunoelectrophoresis of extracts from both sensitive and resistant strains revealed a secondary sialic acid-containing antigen that was electrophoretically different from both the major K-1 antigen and a reference group B meningococcal antigen. This negatively charged secondary antigen was susceptible to Clostridium perfringens neuraminidase degradation and reacted only with IgG whereas the major K-1 antigen reacted only with IgM. This antigen was detected in the extracts of resistant isolates only at 1010 but not at 109 colony-forming units per milliliter. This study demonstrates that (i) the degree of phagocytosis of bacteremic E. coli K-1 isolates is inversely associated with K-1 content, and (ii) more easily phagocytosed (sensitive) K-1 isolates have greater amounts of an additional sialic acid-containing antigen that appears to be unrelated to the previously described O acetyl K-1 antigen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkstén B., Kaijser B. Interaction of human serum and neutrophils with Escherichia coli strains: differences between strains isolated from urine of patients with pyelonephritis or asymptomatic bacteriuria. Infect Immun. 1978 Nov;22(2):308–311. doi: 10.1128/iai.22.2.308-311.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyum A. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4(4):269–274. [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y., Artenstein M. S. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J Exp Med. 1969 Jun 1;129(6):1349–1365. doi: 10.1084/jem.129.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Rey M., Etienne J., Sanborn W. R., Triau R., Cvjetanović B. The immunological responses observed in field studies in Africa with group A meningococcal vaccines. Prog Immunobiol Stand. 1971;5:485–491. [PubMed] [Google Scholar]
- Howard C. J., Glynn A. A. The virulence for mice of strains of Escherichia coli related to the effects of K antigens on their resistance to phagocytosis and killing by complement. Immunology. 1971 May;20(5):767–777. [PMC free article] [PubMed] [Google Scholar]
- KERBY G. P. A comparison of the removal of mucoid and non-mucoid variants of Klebsiella pneumoniae type B from the splanchnic circulating blood of the intact animal. J Immunol. 1950 Mar;64(3):131–137. [PubMed] [Google Scholar]
- Kasper D. L., Winkelhake J. L., Zollinger W. D., Brandt B. L., Artenstein M. S. Immunochemical similarity between polysaccharide antigens of Escherichia coli 07: K1(L):NM and group B Neisseria meningitidis. J Immunol. 1973 Jan;110(1):262–268. [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C., Dunne F. T., Jonssen E. K. Studies on the meningococcal polysaccharides. II. Composition and chemical properties of the group B and group C polysaccharide. J Biol Chem. 1971 Aug 10;246(15):4703–4712. [PubMed] [Google Scholar]
- Orskov F., Orskov I., Sutton A., Schneerson R., Lin W., Egan W., Hoff G. E., Robbins J. B. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J Exp Med. 1979 Mar 1;149(3):669–685. doi: 10.1084/jem.149.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Normann B., Edebo L. Influence of O and K antigens on the surface properties of Escherichia coli in relation to phagocytosis. Acta Pathol Microbiol Scand B. 1979 Apr;87B(2):85–91. doi: 10.1111/j.1699-0463.1979.tb02408.x. [DOI] [PubMed] [Google Scholar]
- Stevens P., Huang S. N., Welch W. D., Young L. S. Restricted complement activation by Escherichia coli with the K-1 capsular serotype: a possible role in pathogenicity. J Immunol. 1978 Dec;121(6):2174–2180. [PubMed] [Google Scholar]
- Stevens P., Young L. S. Quantitative granulocyte chemiluminescence in the rapid detection of impaired opsonization of Escherichia coli. Infect Immun. 1977 Jun;16(3):796–804. doi: 10.1128/iai.16.3.796-804.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk W. C., Verbrugh H. A., Peters R., Van Der Tol M. E., Peterson P. K., Verhoef J. Escherichia coli K antigen in relation to serum-induced lysis and phagocytosis. J Med Microbiol. 1979 Feb;12(1):123–130. doi: 10.1099/00222615-12-1-123. [DOI] [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. II. Contact angles and phagocytosis of encapsulated bacteria before and after opsonization by specific antiserum and complement. J Reticuloendothel Soc. 1972 Nov;12(5):497–502. [PubMed] [Google Scholar]
- Weeke B. Crossed immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:47–56. doi: 10.1111/j.1365-3083.1973.tb03778.x. [DOI] [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]
- Weinstein R., Young L. S. Phagocytic resistance of Escherichia coli K-1 isolates and relationship to virulence. J Clin Microbiol. 1978 Dec;8(6):748–755. doi: 10.1128/jcm.8.6.748-755.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller P. F., Smith A. L., Anderson P., Smith D. H. The role of encapsulation and host age in the clearance of Haemophilus influenzae bacteremia. J Infect Dis. 1977 Jan;135(1):34–41. doi: 10.1093/infdis/135.1.34. [DOI] [PubMed] [Google Scholar]