Abstract
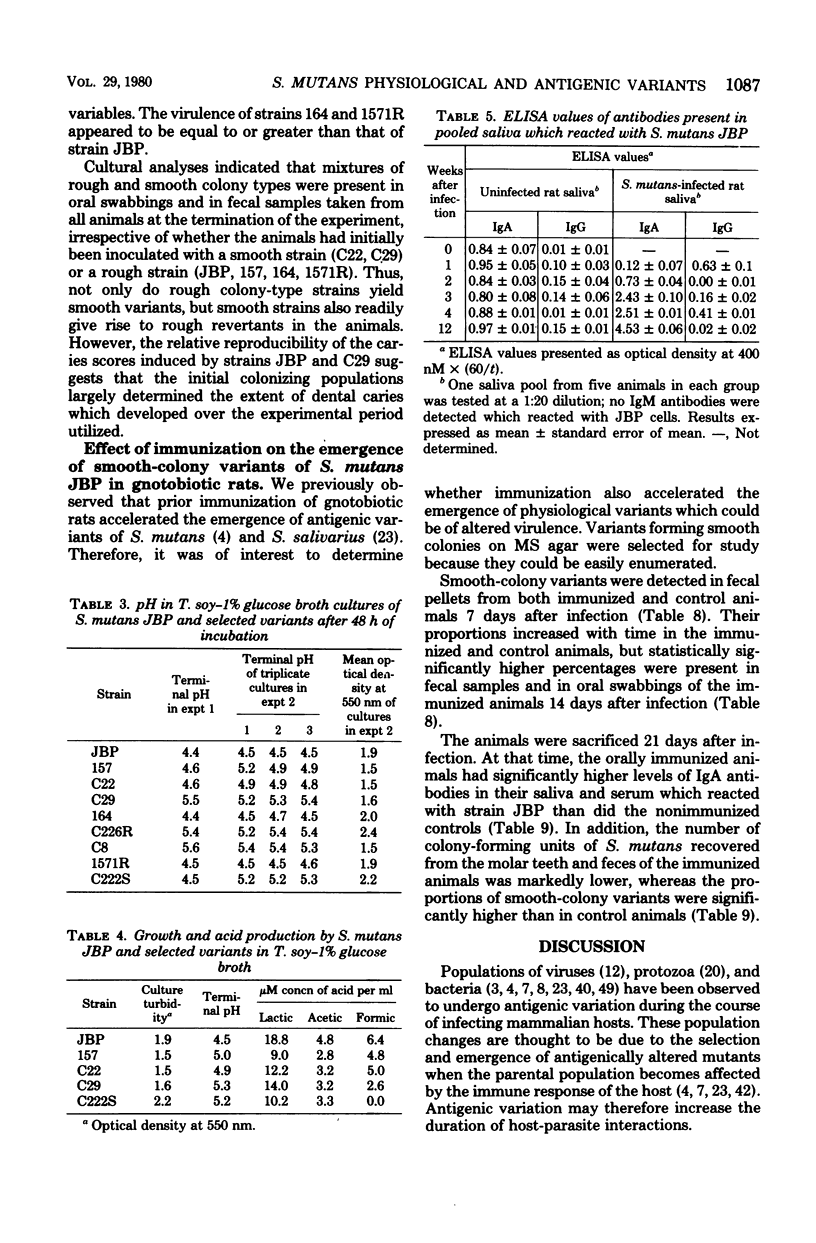
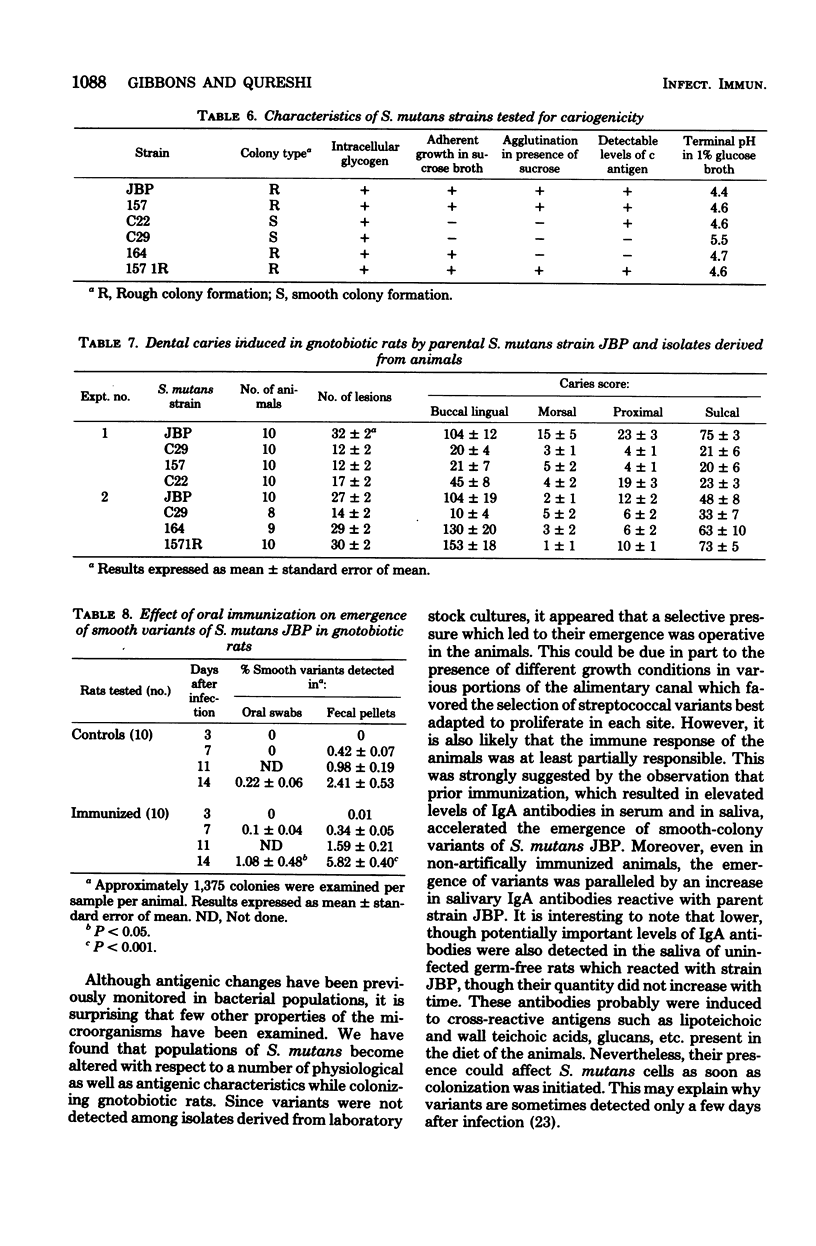
The purpose of this study was to determine if populations of Streptococcus mutans which were undergoing antigenic variation while colonizing gnotobiotic rats concomitantly became altered in physiological characteristics which affected their virulence. S. mutans strain JBP (serotype c), which was freshly isolated from a carious lesion in a 6-year old child, was used to inoculate gnotobiotic rats; uninfected animals served as controls. Substrains were isolated from animals 1, 2, 3, 4, and 12 weeks after infection; samples of pilocarpine-stimulated saliva were also obtained from representative animals for antibody analyses. Isolates derived from stock cultures of strain JBP proved to be homogeneous with respect to all of the physiological characteristics monitored. However, substrains isolated from the animals within 4 weeks after infection were altered with respect to their ability to agglutinate in the presence of sucrose, their ability to form adherent growth in sucrose broth, and the terminal pH attained in glucose broth. Some isolates obtained 12 weeks after infection no longer synthesized detectable levels of c antigen or intracellular glycogen, and they formed atypical smooth colonies on mitis salivarius agar. With an enzyme-linked immunosorbent assay, low levels of immunoglobulin A (IgA) antibodies reactive with whole JBP cells were detected in saliva samples of uninfected control animals at each sampling period; these evidently were induced to antigens contained in the diet of the animals. Significantly higher levels of IgA antibodies were present in saliva samples from animals infected with strain JBP for 3 weeks or longer. Thus, the emergence of antigenic and physiological variants of S. mutans in the animals was paralleled by increased levels of salivary IgA antibodies. The reactivity of salivary IgG with JBP cells was low, and it fluctuated in both groups of animals. No antibodies of the IgM class were detected. When tested in gnotobiotic rats, several variants, including strains which no longer formed typical rough colonies or adherent growth in sucrose broth, proved much less virulent than parental strain JBP in inducing carious lesions. Prior oral immunization, which resulted in higher levels of salivary and serum IgA antibodies reactive with strain JBP, was found to accelerate the emergence of smooth-colony variants in the animals; it was also associated with decreased streptococcal population levels on the teeth and in feces of the rats. It is suggested that part of the mechanism by which artificial immunization leads to a reduction in dental caries development in experimental animals is due to the earlier selection of less virulent streptococcal populations.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BHASKARAN K., GORRILL R. H. A study of antigenic variation in Vibrio cholerae. J Gen Microbiol. 1957 Jun;16(3):721–729. doi: 10.1099/00221287-16-3-721. [DOI] [PubMed] [Google Scholar]
- Bammann L. L., Clark W. B., Gibbons R. J. Impaired colonization of gnotobiotic and conventional rats by streptomycin-resistant strains of Streptococcus mutans. Infect Immun. 1978 Dec;22(3):721–726. doi: 10.1128/iai.22.3.721-726.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bammann L. L., Gibbons R. J. Immunoglobulin A antibodies reactive with Streptococcus mutans in saliva of adults, children, and predentate infants. J Clin Microbiol. 1979 Oct;10(4):538–543. doi: 10.1128/jcm.10.4.538-543.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratthall D., Gibbons R. J. Antigenic variation of Streptococcus mutans colonizing gnotobiotic rats. Infect Immun. 1975 Dec;12(6):1231–1236. doi: 10.1128/iai.12.6.1231-1236.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratthall D., Gibbons R. J. Changing agglutination activities of salivary immunoglobulin A preparations against oral streptococci. Infect Immun. 1975 Mar;11(3):603–606. doi: 10.1128/iai.11.3.603-606.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil L. B., Schurig G. G., Bier P. J., Winter A. J. Bovine veneral vibriosis: antigenic variation of the bacterium during infection. Infect Immun. 1975 Feb;11(2):240–244. doi: 10.1128/iai.11.2.240-244.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS R. T., MERGENHAGEN S. E. OCCURRENCE OF NATURAL ANTIBACTERIAL ANTIBODY IN HUMAN PAROTID FLUID. Proc Soc Exp Biol Med. 1965 Jul;119:815–819. doi: 10.3181/00379727-119-30309. [DOI] [PubMed] [Google Scholar]
- Edwardsson S. The caries-inducing property of variants of Streptococcus mutans. Odontol Revy. 1970;21(2):153–157. [PubMed] [Google Scholar]
- Edén C. S., Hanson L. A., Jodal U., Lindberg U., Akerlund A. S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet. 1976 Sep 4;1(7984):490–492. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Fitzgerald D. B., Stevens R., Fitzgerald R. J., Mandel I. D. Comparative cariogenicity of streptococcus mutans strains isolated from caries active and caries resistant adults. J Dent Res. 1977 Aug;56(8):894–894. doi: 10.1177/00220345770560080901. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Tanzer J. M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):189–196. doi: 10.1128/iai.10.1.189-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., SOCRANSKY S. S. Intracellular polysaccharide storage by organisms in dental plaques. Its relation to dental caries and microbial ecology of the oral cavity. Arch Oral Biol. 1962 Jan-Feb;7:73–79. doi: 10.1016/0003-9969(62)90050-x. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gold O. G., Jordan H. V., Van Houte J. A selective medium for Streptococcus mutans. Arch Oral Biol. 1973 Nov;18(11):1357–1364. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- Gray A. R. Antigenic variation in a strain of Trypanosoma brucei transmitted by Glossina morsitans and G. palpalis. J Gen Microbiol. 1965 Nov;41(2):195–214. doi: 10.1099/00221287-41-2-195. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta. 1967 Oct;11(2):131–152. [PubMed] [Google Scholar]
- Hanson L. A., Ahlstedt S., Carlsson B., Kaijser B., Larsson P., Baltzer I. M., Akerlund A. S., Edén C. S., Svennerholm A. M. Secretory IgA antibodies to enterobacterial virulence antigens: their induction and possible relevance. Adv Exp Med Biol. 1978;107:165–176. doi: 10.1007/978-1-4684-3369-2_20. [DOI] [PubMed] [Google Scholar]
- Howell T. H., Spinell D. M., Gibbons R. J. Antigenic variation in populations of Streptococcus salivarius isolated from the human mouth. Arch Oral Biol. 1979;24(5):389–397. doi: 10.1016/0003-9969(79)90107-9. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Otake S., Hirasawa M., Williams K., Kiyoyono H., McGhee J. R., Shiota T. Virulence of Streptococcus mutans: revertants of mutant C4. Infect Immun. 1980 Jan;27(1):25–31. doi: 10.1128/iai.27.1.25-31.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYES P. H. Dental caries in the molar teeth of rats. II. A method for diagnosing and scoring several types of lesions simultaneously. J Dent Res. 1958 Nov-Dec;37(6):1088–1099. doi: 10.1177/00220345580370060901. [DOI] [PubMed] [Google Scholar]
- KRAUS F. W., KONNO J. THE SALIVARY SECRETION OF ANTIBODY. Ala J Med Sci. 1965 Jan;2:15–22. [PubMed] [Google Scholar]
- Kelstrup J., Richmond S., West C., Gibbons R. J. Fingerprinting human oral streptococci by bacteriocin production and sensitivity. Arch Oral Biol. 1970 Dec;15(12):1109–1116. doi: 10.1016/0003-9969(70)90001-4. [DOI] [PubMed] [Google Scholar]
- LANCEFIELD R. C., PERLMANN G. E. Preparation and properties of a protein (R antigen) occurring in streptococci of group A, type 28 and in certain streptococci of other serological groups. J Exp Med. 1952 Jul;96(1):83–97. doi: 10.1084/jem.96.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Challacombe S. J., Caldwell J. An immunological investigation into the prevention of caries in deciduous teeth of rhesus monkeys. Arch Oral Biol. 1975 May-Jun;20(5-6):305–310. doi: 10.1016/0003-9969(75)90019-9. [DOI] [PubMed] [Google Scholar]
- Linzer R., Gill K., Slade H. D. Chemical composition of Streptococcus mutans type c antigen: comparison to type a, b, and d antigens. J Dent Res. 1976 Jan;55:A109–A115. doi: 10.1177/002203457605500103011. [DOI] [PubMed] [Google Scholar]
- Masuda N., Tsutsumi N., Sobue S., Hamada S. Longitudinal survey of the distribution of various serotypes of Streptococcus mutans in infants. J Clin Microbiol. 1979 Oct;10(4):497–502. doi: 10.1128/jcm.10.4.497-502.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., McGhee J. R., Babb J. L. Effective immunity to dental caries: dose-dependent studies of secretory immunity by oral administration of Streptococcus mutans to rats. Infect Immun. 1978 Jan;19(1):217–224. doi: 10.1128/iai.19.1.217-224.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., Shiota T., Ikeda T., Navia J. M., McGhee J. R. Virulence of Streptococcus mutans: biochemical and pathogenic characteristics of mutant isolates. Proc Soc Exp Biol Med. 1975 Nov;150(2):498–502. doi: 10.3181/00379727-150-39064. [DOI] [PubMed] [Google Scholar]
- Quick J. D., Goldberg H. S., Sonnenwirth A. C. Human antibody to Bacteroidaceae. Am J Clin Nutr. 1972 Dec;25(12):1351–1356. doi: 10.1093/ajcn/25.12.1351. [DOI] [PubMed] [Google Scholar]
- Quinn R. W., Lowry P. N. Some characteristics of nontypable group A streptococci. Yale J Biol Med. 1972 Dec;45(6):572–583. [PMC free article] [PubMed] [Google Scholar]
- Qureshi J. V., Goldner M., Riche W. H., Hargreaves J. A. Streptococcus mutans serotypes in young schoolchildren. Caries Res. 1977;11(3):141–152. doi: 10.1159/000260260. [DOI] [PubMed] [Google Scholar]
- RANTZ L. A., RANDALL E. Use of autoclaved extracts of hemolytic streptococci for serological grouping. Stanford Med Bull. 1955 May;13(2):290–291. [PubMed] [Google Scholar]
- ROBINET H. G. Relationship of host antibody to fluctuations of Escherichia coli serotypes in the human intestine. J Bacteriol. 1962 Nov;84:896–901. doi: 10.1128/jb.84.5.896-901.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B., Miller C. E. Progressive changes of Vibrio serotypes in germ-free mice infected with Vibrio cholerae. J Bacteriol. 1969 Sep;99(3):688–695. doi: 10.1128/jb.99.3.688-695.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro J. P., Muirhead P. A. Quantitative method for the gas chromatographic analysis of short-chain monocarboxylic and dicarboxylic acids in fermentation media. Appl Microbiol. 1975 Mar;29(3):374–381. doi: 10.1128/am.29.3.374-381.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurig G. G., Hall C. E., Corbell L. B., Duncan J. R., Winter A. J. Bovine veneral vibriosis: cure of genital infection in females by systemic immunization. Infect Immun. 1975 Feb;11(2):245–251. doi: 10.1128/iai.11.2.245-251.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Hageage G. J., Jr, Larson R. H. Variable experiences in immunization of rats against Streptococcus mutans-associated dental caries. Arch Oral Biol. 1973 Nov;18(11):1425–1439. doi: 10.1016/0003-9969(73)90117-9. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- van Houte J., Burgess R. C., Onose H. Oral implantation of human strains of Streptococcus mutans in rats fed sucrose or glucose diets. Arch Oral Biol. 1976;21(9):561–564. doi: 10.1016/0003-9969(76)90023-6. [DOI] [PubMed] [Google Scholar]
- van Houte J., Winkler K. C., Jansen H. M. Iodophilic polysaccharide synthesis, acid production and growth in oral streptococci. Arch Oral Biol. 1969 Jan;14(1):45–61. doi: 10.1016/0003-9969(69)90020-x. [DOI] [PubMed] [Google Scholar]



