Abstract
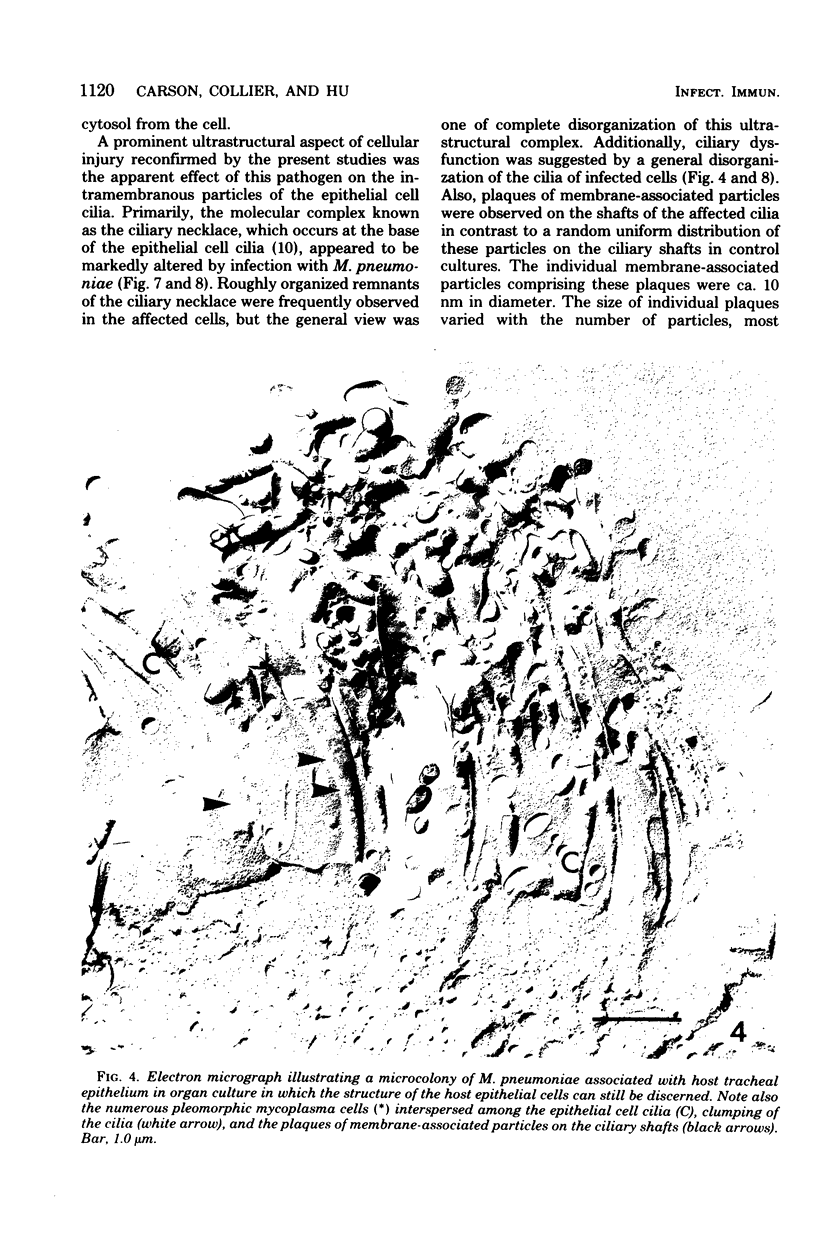
The ultrastructural organization of Mycoplasma pneumoniae membranes and spatial relationships of this pathogen to epithelial cells in tracheal organ cultures were examined ultrastructurally by freeze-fracture techniques. Areas of morphologically distinct cell membrane variability characterized by membrane blebs and altered distributions of membrane associated particles were observed in replicas of M. pneumoniae cells. Inspection of the host tracheal epithelium demonstrated the alignment of M. pneumoniae to the epithelium with an accompanying deterioration in the integrity of the lumenal surface membranes and subsequent loss of the epithelial cell cytosol. Ciliary dysfunction was suggested by the observation of ciliary lesions and of disorganized epithelial cell cilia. The methodology used in these studies has permitted a new perspective of host-pathogen interactions at both the cellular and subcellular levels in tracheal organ cultures. These studies may also illustrate ultrastructural correlates of the alteration of host macromolecular synthesis in experimental M. pneumoniae infection.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein-Ziv R. Cell division in Mycoplasma gallisepticum. Can J Microbiol. 1969 Oct;15(10):1125–1128. doi: 10.1139/m69-204. [DOI] [PubMed] [Google Scholar]
- Carson J. L., Collier A. M., Clyde W. A., Jr Ciliary membrane alterations occurring in experimental Mycoplasma pneumoniae infection. Science. 1979 Oct 19;206(4416):349–351. doi: 10.1126/science.113877. [DOI] [PubMed] [Google Scholar]
- Carson J. L., Collier A. M., Hu S. S. Ultrastructural studies of hamster tracheal epithelium in vivo and in vitro. J Ultrastruct Res. 1980 Jan;70(1):70–81. doi: 10.1016/s0022-5320(80)90023-4. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr Appearance of Mycoplasma pneumoniae in lungs of experimentally infected hamsters and sputum from patients with natural disease. Am Rev Respir Dis. 1974 Dec;110(6):765–773. doi: 10.1164/arrd.1974.110.6P1.765. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A. Relationships Between Mycoplasma pneumoniae and Human Respiratory Epithelium. Infect Immun. 1971 May;3(5):694–701. doi: 10.1128/iai.3.5.694-701.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny F. W., Clyde W. A., Jr, Glezen W. P. Mycoplasma pneumoniae disease: clinical spectrum, pathophysiology, epidemiology, and control. J Infect Dis. 1971 Jan;123(1):74–92. doi: 10.1093/infdis/123.1.74. [DOI] [PubMed] [Google Scholar]
- Gilula N. B., Satir P. The ciliary necklace. A ciliary membrane specialization. J Cell Biol. 1972 May;53(2):494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green F., 3rd, Hanson R. P. Ultrastructure and capsule of Mycoplasma meleagridis. J Bacteriol. 1973 Nov;116(2):1011–1018. doi: 10.1128/jb.116.2.1011-1018.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Alterations in the metabolism of hamster tracheas in organ culture after infection by virulent Mycoplasma pneumoniae. Infect Immun. 1975 Apr;11(4):704–710. doi: 10.1128/iai.11.4.704-710.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor H. Use of freeze-etching in the study of biological ultrastructure. Int Rev Exp Pathol. 1966;5:179–216. [PubMed] [Google Scholar]
- Muse K. E., Powell D. A., Collier A. M. Mycoplasma pneumoniae in hamster tracheal organ culture studied by scanning electron microscopy. Infect Immun. 1976 Jan;13(1):229–237. doi: 10.1128/iai.13.1.229-237.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillack T. W., Carter R., Razin S. Native and reformed Mycoplasma laidlawii membranes compared by freeze-etching. Biochim Biophys Acta. 1970;219(1):123–130. doi: 10.1016/0005-2736(70)90067-2. [DOI] [PubMed] [Google Scholar]
- Tourtellotte M. E., Zupnik J. S. Freeze-fractured Acholeplasma laidlawii membranes: nature of particles observed. Science. 1973 Jan 5;179(4068):84–86. doi: 10.1126/science.179.4068.84. [DOI] [PubMed] [Google Scholar]
- Wilson M. H., Collier A. M. Ultrastructural study of Mycoplasma pneumoniae in organ culture. J Bacteriol. 1976 Jan;125(1):332–339. doi: 10.1128/jb.125.1.332-339.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]










