Abstract
Red blood cells (RBCs) are key players in systemic oxygen transport. RBCs respond to in vitro hypoxia through the so-called oxygen-dependent metabolic regulation, which involves the competitive binding of deoxyhemoglobin and glycolytic enzymes to the N-terminal cytosolic domain of band 3. This mechanism promotes the accumulation of 2,3-DPG, stabilizing the deoxygenated state of hemoglobin, and cytosol acidification, triggering oxygen off-loading through the Bohr effect. Despite in vitro studies, in vivo adaptations to hypoxia have not yet been completely elucidated.
Within the framework of the AltitudeOmics study, erythrocytes were collected from 21 healthy volunteers at sea level, after exposure to high altitude (5260m) for 1, 7 and 16days, and following reascent after 7days at 1525m. UHPLC-MS metabolomics results were correlated to physiological and athletic performance parameters.
Immediate metabolic adaptations were noted as early as a few hours from ascending to >5000m, and maintained for 16 days at high altitude. Consistent with the mechanisms elucidated in vitro, hypoxia promoted glycolysis and deregulated the pentose phosphate pathway, as well purine catabolism, glutathione homeostasis, arginine/nitric oxide and sulphur/H2S metabolism.
Metabolic adaptations were preserved one week after descent, consistently with improved physical performances in comparison to the first ascendance, suggesting a mechanism of metabolic memory.
Keywords: red blood cell, mass spectrometry, metabolomics, metabolic linkage, nitric oxide, hydrogen sulfide
Introduction
Understanding systemic adaptations to hypoxia is a challenging task, one of relevance to a broad community of researchers and clinicians involved in cardiovascular research,1 pulmunology2, transfusion medicine,3 and intesive care medicine4. Of note, unraveling the mechanisms of adaptation to hypoxia might influence our understanding of evolutionary adaptations as extreme as mammalian hibernation,5 a poorly understood phenomenon that holds potential clinical, military or space-travel translational applications6. Other than for clinical/research purposes, understanding systemic adaptations to hypoxia might also influence the daily lives of millions of healthy people around the world. Approximately 140 million people live permanently, or travel to high altitudes (>2500 m) in North, Central and South America, East Africa, and Asia.7 Many people successfully adjust to the hypoxic environment at very high altitudes (~5000 m), where oxygen pressures are about half of those registered at sea level.
Despite decades of strides in the field8–14, the current mechanistic understanding of human in vivo adaptations to hypoxia is still incomplete. Undoubtedly, red blood cells (RBCs) play a clear role in adaptations to hypoxia, in line with their vital role in oxygen transport and delivery.8–14 Increases in red cell volume (RCV) and total hemoglobin mass (Hbmass) are observed as early as one or two weeks after exposure to high altitude, even though these adaptations are eventually lost following descent to low altitude.14,15 Besides, while hypoxia can induce systemic increase of erythropoietin (EPO) levels within hours of hypobaric hypoxia16, EPO-stimulated production of mature RBCs from the bone marrow can take days to occur.17
Cellular adaptations to hypoxia also involve the stabilization of hypoxia-inducible factors (HIFs), a family of transcriptional factors18 involved in metabolic regulation, as it is increasingly emerging in cancer19 and pulmonary hypertension.20 HIF degradation is mediated through HIF hydroxylation by O2-sensing protein hydroxylases (PHDs).19 Mutations of PHD221 results in decreased degradation of HIF1α, that in turn transcriptionally regulates numerous metabolic enzymes22, thereby contributing to adaptive metabolic responses to hypoxia such as increased erythropoiesis21 in patients carrying the mutation. At the same time, hypoxia-induced uncoupling of the electron transport chain promotes increases in the levels of Krebs cycle intermediates, which in turn promote the stabilization of HIF1α through the direct inhibition of PHDs, suggesting a crosstalk between metabolic adaptation and gene expression phenotypes under hypoxia.23
Decades of laboratory studies aimed at understanding RBC responses to deoxygenation and hypoxia have fostered great advances in structural and functional biochemistry, introducing the concept of allosteric modulation24. Over the years, structural and functional evidence has been produced about the hypoxia-dependent promotion of hemoglobin oxygen off-loading through the stabilization of the deoxygenated tense state (T) by negatively charged high phosphate compounds adenosine triphosphate (ATP) and 2,3-diphosphoglycerate (DPG)8. In the last twenty years, the model has been expanded as to introduce the concept of the “transport metabolon”, which involves band 3. As the most abundant RBC membrane protein (1×106 copies/cell), band 3 modulates CO2 gas transport in erythrocytes through the so-called “chloride shift” (HCO3−/Cl− exchange), thereby contributing to pH homeostasis and oxygen off-loading by promoting the “Bohr effect”.25 The “transport metabolon” model is based on the observation that the N-terminal cytosolic domain of band 3, which contains numerous acidic residues, might stabilize deoxyhemoglobin through direct binding.26–29 However, the N-terminal region of band 3 also serves as a docking site for key glycolytic enzymes, including phosphofructokinase (PFK), aldolase (ALDO) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). These enzymes are functionally inhibited by binding to band 3 at high oxygen saturation, thereby promoting late glycolytic blockade and a shift towards the anti-oxidant NADPH-generating pentose phosphate pathway (PPP). 26–29 Binding of deoxyhemoglobin to band 3 promotes the displacement and activation of late glycolytic enzymes, thus favoring glycolysis.26–29 According to this model, 26–29 as much as 92% of glucose is catabolized through the classic Embden-Meyerhoff glycolytic pathway under normoxia, while anoxia triggers consumption of as much as 90% of glucose via the PPP. This model has been supported by in vitro evidence30 and in silico prediction based on metabolomics data of ex vivo aging RBCs under anaerobic conditions31,32. However, evidence of in vivo RBC metabolic adaptations to hypoxia has not been hitherto produced.
Here we hypothesize that exposure to high-altitude hypoxia triggers dramatic metabolic modulation of RBCs in humans. These metabolic adaptations might underlie adaptations to high altitude hypoxia, a phenomenon that is partially retained upon later re-ascent.33 RBC metabolic phenotypes have been here correlated to physiological tests, as to understand whether metabolic adaptations might at least partially explain retention of improved performances upon adjustment to hypoxia during second ascents.
Methods
The study was performed as part of the AltitudeOmics research program, as previously reported15,33–39. Twenty-one healthy volounteers (12 males and nine females, 19–23 years - Supplementary Table 1) were enrolled upon written consent, in agreement with the Declaration of Helsinki. The study was approved by the Institutional Review Boards of the Universities of Colorado and Oregon and by the Human Research Protection Office of the U.S. Department of Defense. Exclusion criteria included: being born at >1500 m; having traveled to altitudes >1000 m in the past three months (including air travel); using prescription medications; smoking; self or familial history of migraine; known hematologic or cardiovascular abnormality; pulmonary function or diffusion capacity for carbon monoxide <90% of predicted.
Timeline
Subjects were studied near sea level (SL) (130 m, average PB = 749 mmHg), and over three study periods at Mt Chacaltaya, Bolivia (5260 m; average PB = 406 mmHg; fed ad libitum), on the first, seventh and sixteenth days at 5260 m (ALT1, ALT7, ALT16; n=20), and again upon reascent to 5260 m, after 7 (n = 14) days at low altitude (POST). Subjects breathed supplemental oxygen (2 L/min, nasal cannula or mask) during the drive for the first ascent to 5260 m.
Blood processing and metabolomics extraction
Whole blood was drawn from an antecubital venous catheter and immediately processed to sort plasma and cell components at the same time of the day (noon) at SL, and on ALT1, 7, 16 and POST at high altitude. RBCs were snap frozen in liquid nitrogen and stored at −80°C prior to metabolomics analyses. RBCs (100 μl) were extracted in lysis/extraction buffer (methanol:acetonitrile:water 5:3:2, −20°C) at 1:9 dilution, as previously reported40. Samples were vortexed at 4°C for 30 min and then centrifuged at 10,000g for 15min at 4°C to pellet proteins and collect the supernatants for metabolomics analyses.
Metabolomics analysis
RBC extracts (10 μl) were injected into an UHPLC system (Ultimate 3000, Thermo, San Jose, CA, USA) and run on a a Kinetex XB-C18 column (150×2.1 mm i.d., 1.7 μm particle size – Phenomenex, Torrance, CA, USA), as reported41. MS analyses through a QExactive mass spectrometer (Thermo, San Jose, CA, USA) and metabolite identification through Maven42 (Princeton, NJ, USA), the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database, and a library of >800 standard compounds (SIGMA Aldrich, St. Louis, MO, USA; MLSMS, IROATech, Bolton, MA, USA) were performed as reported40,41.
Measurements of nitrite, nitrate, S-NO and H2S
Methods for the measurement of nitrate (NO3−) and nitrite (NO2−) and H2S were performed as extensively reported.43,44
Statistical Analysis
Integrated peak area values were exported into Excel (Microsoft, Redmond, CA, USA) for statistical analysis including T-Test and ANOVA (significance threshold for p-values < 0.05; false discovery rate cutoffs set to 0.01 for initial screening and 0.05 for time point-specific comparisons) and partial least square discriminant analysis (PLS-DA), calculated through the macro MultiBase (freely available at www.NumericalDynamics.com). To exclude overfitting of PLS-DA elaboration, we repeated the clustering analysis by performing random permutations. Hierarchical clustering analysis (HCA) was performed through the software GENE-E (Broad Institute, Cambridge, MA, USA). Pearson’s correlations and XY graphs were calculated and plotted through GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA). Figure panels were assembled through Photoshop CS5 (Adobe, Mountain View, CA, USA).
Results
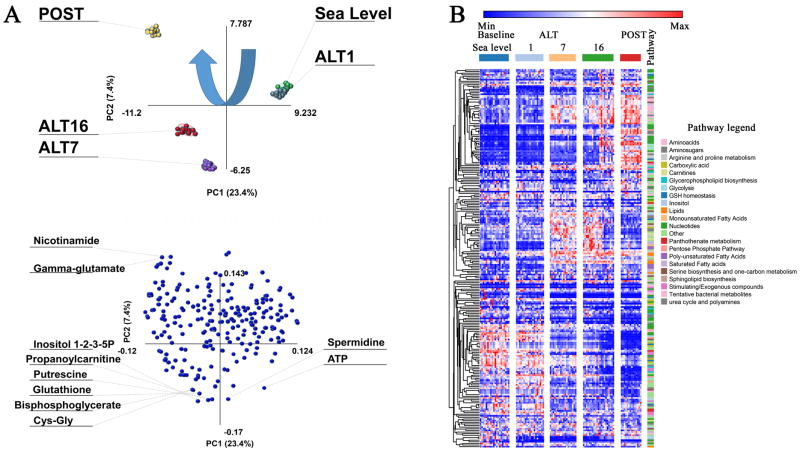
A total of 229 metabolites were monitored in RBC extracts from 21 subjects enrolled in this study at sea leavel, or 1, 7 and 16 days at high altitude, or upon ascending for a second time to high altitude after 7 days at low altitude (SL, ALT1, 7 and 16 and POST time points in Supplementary Table S1). Relative ion counts (integrated peak areas of extracted ion chromatograms for each metabolite) are provided, together with KEGG pathway compound IDs, pathway names (color coded, in agreement with the legend at the bottom of the table), the experimental mass to charge ratios and retention times, and the polarity in which each metabolite has been assayed. Elaboration files for PLS-DA, HCA and ANOVA are included in Supplementary Table S1 and S2, and the results of both statistical analyses are plotted in Figure 1.A and B, respectively. PLS-DA of RBC metabolic profiles clearly discriminated the sample groups across two main principal components (PCs) (Figure 1.A). The top ten metabolites (loading variables) contributing to the PLS-DA clustering pattern are highlighted in Figure 1.B, and include metabolites involved in energy metabolism (adenosine triphosphate – ATP, bisphosphoglcyerate – BPG), glutathione homeostasis (glutathione - GSH, cysteine-glycine, gamma-glutamate), and polyamines (spermidine, putrescine). HCA highlighted three main trends for metabolite relative abundances during adaptation, including (i) metabolites that accumulate during adaptation (blue to red); (ii) metabolites that transiently increase (blue-red-blue); and (iii) metabolites that decrease following exposure to hypoxia (red to blue - from top to bottom, left to right in Figure 1.B, extended version in Supplementary Figure S1).
Figure 1. Partial least-square discriminant analysis (PLS-DA) and hierarchical clustering analysis (HCA) of metabolomics data from AltitudeOmics red blood cells.
In A, PLS-DA of red blood cells metabolomics data from the volunteers involved in the AltitudeOmics study, either collected at sea level, after one, seven or sixteen days at high altitude (ALT1, 7 and 16, respectively), or following volunteer reascending to the mountain 7 days after descending to 1525m. In the top panel each node represents a different sample. In the bottom panel, each node represents a metabolite (variable) in the loading plot. Top ten metabolites with the highest loadings along principal components 1 and 2 (PC1 and PC2) are shown. Percentages of variances are provided for each component.
In B, HCA (1-Pearson’s correlation) of metabolites in each sample across each time point are plotted as heat maps. Z-score normalizations have bene performed intra-row and values are color coded from blue to red (low to high). Pathways are color coded in the right hand legend. An extended version of this panel, also including metabolite and sample names is provided in Supplementary Figure 1.
To ease data interpretation, metabolites showing statistically significant changes (q<0.05 ANOVA) in at least one time point compared to SL measurements were grouped by pathway in Figures 2–5, including (i) glycolysis and pentose phosphate pathway (PPP) (Figure 2); (ii) nitrogen metabolism and purine homeostasis (Figure 3); (iii) amino acid metabolism, GSH homeostasis and transamination (Figure 4); (iv) sulphur and arginine metabolism (Figure 5).
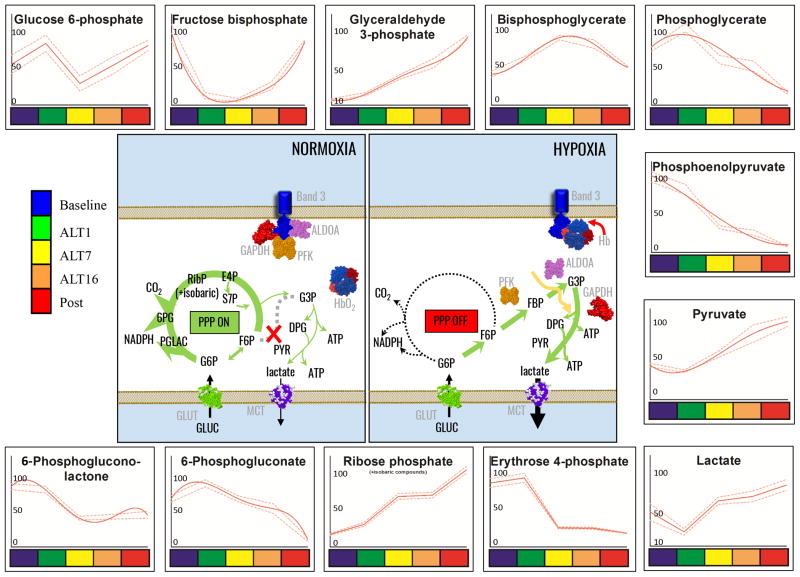
Figure 2. Glycolysis and pentose phosphate pathway in RBC AltitudeOmics samples.
Glycolytic and Pentose Phosphate Pathway metabolites from RBC AltitudeOmics samples are graphed as interpolation curves (solid red line) ± standard deviations (gaped red lines) across each time point, color coded as indicated in the left hand legend. In the center, the figure schematizes the expected effect of oxygen-dependent metabolic modulation through competitive inhibitory binding of glycolytic enzyme and deoxyhemoglobin to the N-terminal cytosolic domain of band 3. In each graph, the y axis indicates integrated peak areas normalized against the highest reading at any time point.
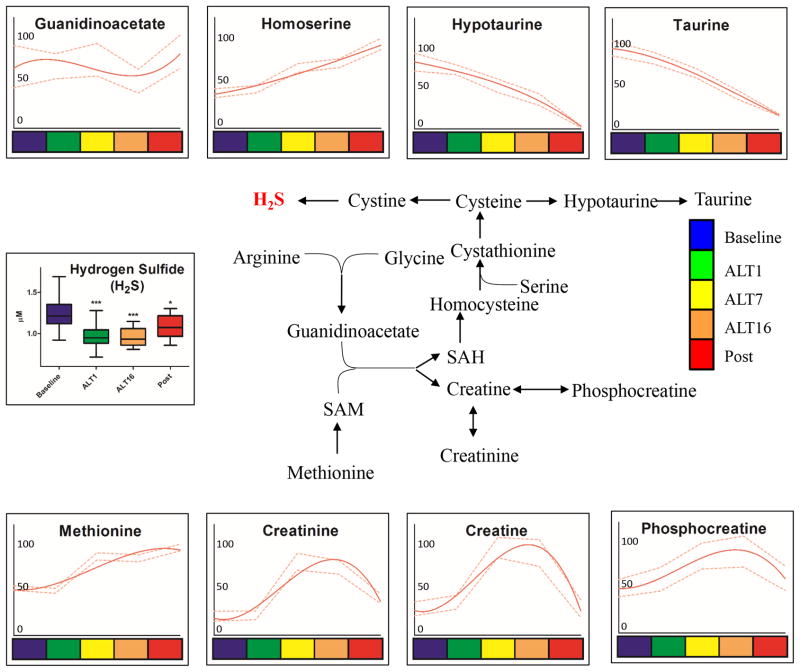
Figure 5. Sulphur and arginine metabolic pathways in RBC AltitudeOmics samples.
Sulphur and arginine pathways (pathway schematized in the center) metabolites from RBC AltitudeOmics samples are graphed as interpolation curves (solid red line) ± standard deviations (gaped red lines) across each time point, color coded as indicated in the right hand legend. In each graph, the y axis indicates integrated peak areas normalized against the highest reading at any time point.
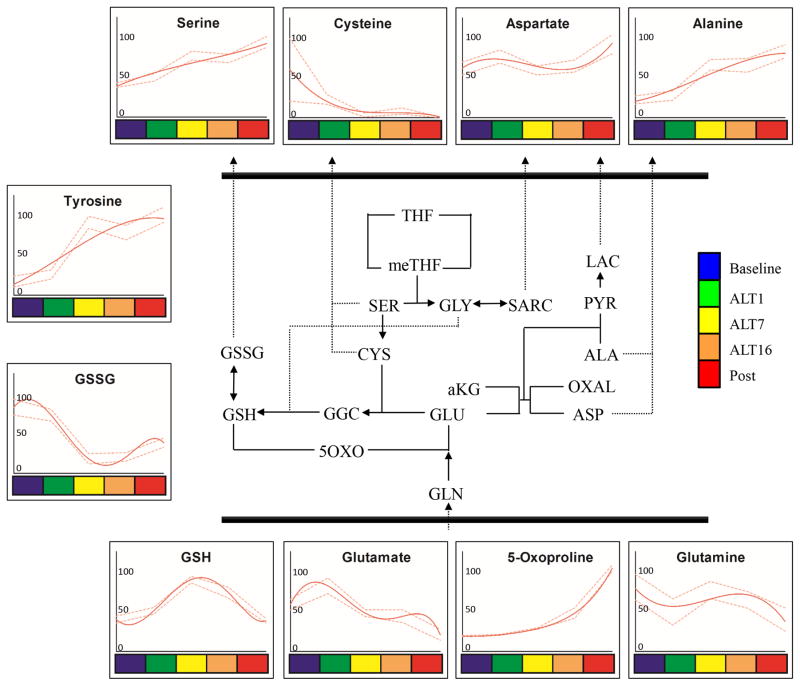
Figure 3. Glutathione homeostasis and transamination pathways in RBC AltitudeOmics samples.
Glutathione homeostasis and transamination pathways (pathway schematized in the center) metabolites from RBC AltitudeOmics samples are graphed as interpolation curves (solid red line) ± standard deviations (gaped red lines) across each time point, color coded as indicated in the right hand legend. In each graph, the y axis indicates integrated peak areas normalized against the highest reading at any time point.
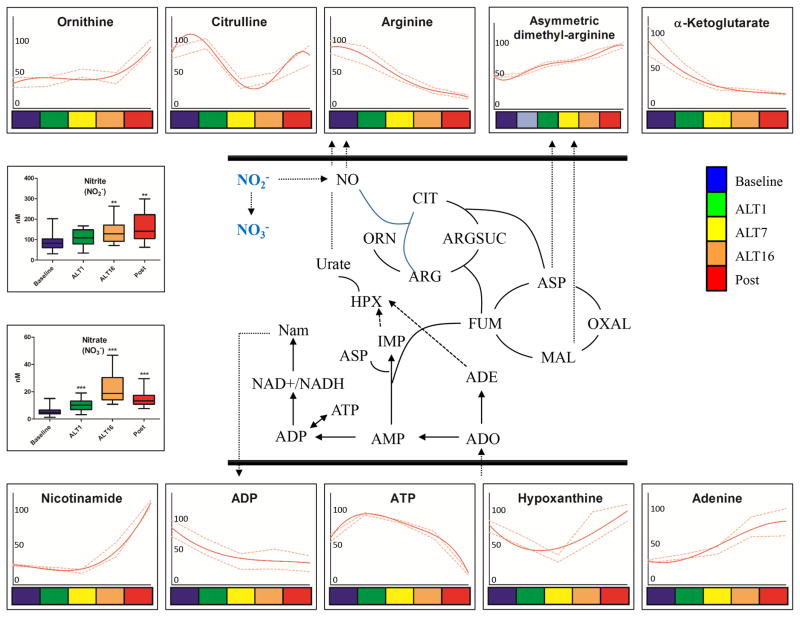
Figure 4. Nitric oxide and purine homeostasis pathways in RBC AltitudeOmics samples.
Nitric oxide, urea cycle and purine homeostasis (pathway schematized in the center) metabolites from RBC AltitudeOmics samples are graphed as interpolation curves (solid red line) ± standard deviations (gaped red lines) across each time point, color coded as indicated in the right hand legend. In each graph, the y axis indicates integrated peak areas normalized against the highest reading at any time point.
Glycolysis and Pentose Phosphate Pathway
Glycolytic precursor glucose 6-phosphate accumulated from day 1 to 7, and decreased again from day 7 to 16. On the other hand, late glycolytic end products downstream of triose phosphates (glyceraldehyde 3-phosphate) accumulated significantly on day 1. Decreases in phosphoglycerate and phosphoenolpyruvate and increases in pyruvate and lactate are suggestive of rapid fluxing through glycolysis in response to hypoxia (Figure 2). In parallel, we observed decreases in the levels of oxidative phase metabolites of the PPP (6-phosphogluconolactone and 6-phosphogluconate –Figure 1).
Amino acids, glutathione homeostasis and transamination
Despite depression of PPP (Figure 2), hypoxia promoted GSH accumulation and oxidized glutathione (GSSG) depletion, consistent with reduced oxidative stress or increased de novo biosynthesis (also supported by the progressive depletion of GSH precursors glutamine/glutamate and cysteine – Figure 3). Glutamate depletion might be influenced by increased transamination of pyruvate (an accumulating byproduct of glycolysis – Figure 2) into alanine (Figure 3).
Nitrogen metabolism
Partially functional urea cycle metabolism is present in mitochondria-devoid RBCs,45–47 as well as a functional nitric oxide synthase48, that competes with arginase for the substrate L-arginine to generate nitric oxide (NO) under ischemic/reperfusion conditions in RBCs49. Of note, in human lung endothelial cells, arginase is upregulated by hypoxia50. Arginine was consumed without accumulation of urea cycle intermediates (citrulline levels oscillated after early significant increases at ALT1, while ornithine levels increased significantly only in POST samples). At the same time, polyamine accumulation was observed transiently at ALT7 (spermidine, spermine). Asymmetric dimethyl-arginine increased after one week and sixteen days (Figure 4). Early arginine consumption, polyamine accumulation at intermediate time points and urea cycle activation after desecent/renascent were observed (Figure 4). Nitrite and nitrate levels increased at ALT1 and ALT16 in comparison to baseline sea level (p<0.01 and 0.001, respectively - Figure 4). Higher than baseline nitrite and nitrate levels were retained after reascending.
Altered purine homeostasis was also mirrored by the significant accumulation of adenine, adenosine, hypoxanthine and nicotinamide proportionally to the duration of exposure to high altitude (Figure 4).
Arginine and sulphur metabolism
Arginine consumption could also be tied to the observed increase in creatine metabolism, as confirmed by the accumulation of creatine, creatinine and phosphocreatine before descent after ALT16 (Figure 5). While arginine catabolism might contribute to nitric oxide (NO) homeostasis, additional vasodilation mechanisms can possibly be explained by decreased levels of taurine/hypotaurine (Figure 5) together with cysteine consumption (Figure 4) to mirror altered function of H2S-generating pathways.51 Direct measurements of hydrogen sulfide (H2S) showed significant decreases after exposure to high altitude hypoxia, and H2S levels remained significantly lower than baseline values during the second reascent (p<0.0001 – Figure 5).
Correlation with physiological parameters
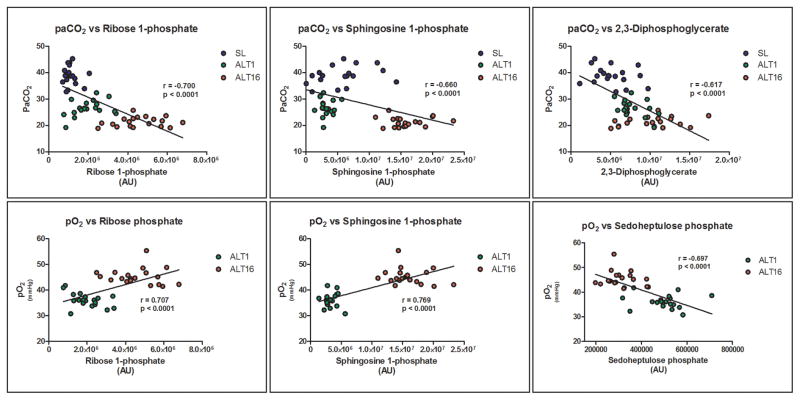
Within the framework of the AltitudeOmics project, physiological, athletic performance and reaction time tests were performed at either sea level, 1 and 16 days at high altitude.15,33–39 Here we correlated raw metabolomics data for each metabolite in each biological replicate at SL, ALT1 and 16 to other data available for each subject (Supplementary Table S3). Linear correlation values (r) were used to perform HCA (Supplementary Figure S2) with the goal of highlighting a core set of metabolites and physiological/athletic parameters that show strong correlations (> |0.6|). The strongest correlations were selected and plotted in Figure 6, and highlight a high correlation of metabolite levels to gaseous measurements (PaO2, PaCO2) especially for the PPP final product ribose phosphate and sphingosine 1-phosphate.
Figure 6. Linear correlations of metabolite levels and physiological parameters.
Physiological parameters assayed in AltitudeOmics volounteers were correlated to metabolite levels at matched time points (sea level – SL, altitude 1 and 16 – ALT1 and ALT16), color-coded as per the right hand legend. Linear correlations and statistical significance are shown for each panel.
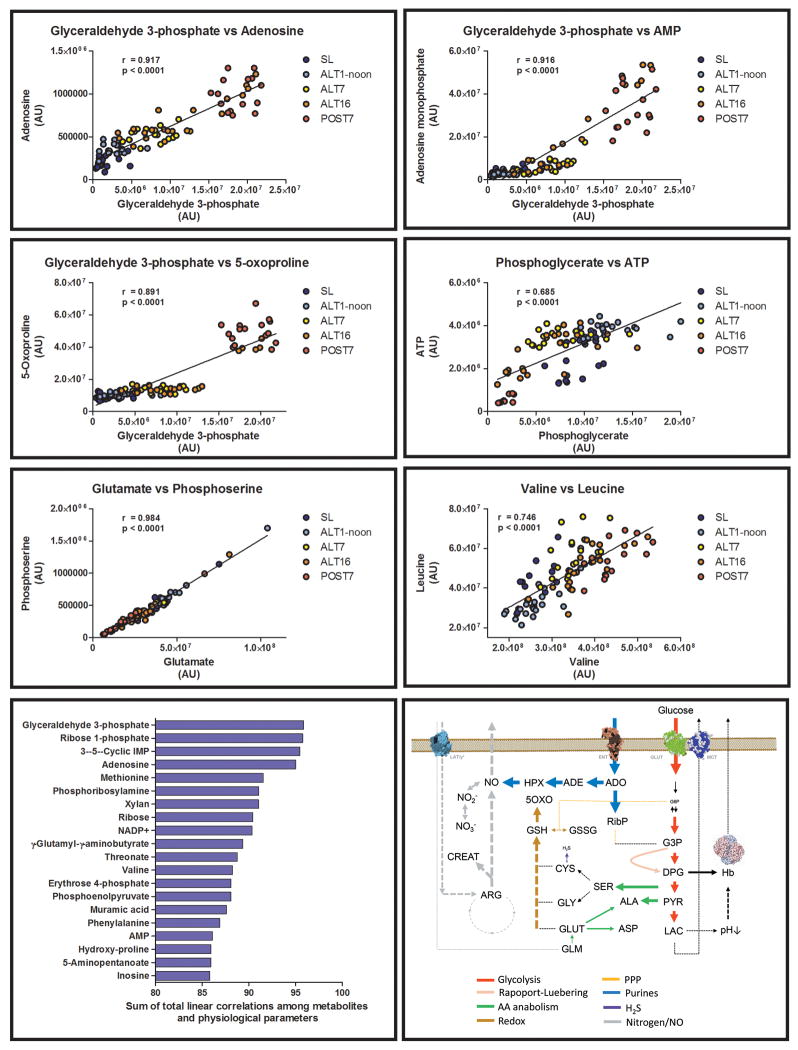
Metabolite levels were correlated among each other at each time point (including SL, ALT1, 7 and 16 and POST - Figure 7). As a result, highest significant correlations (p<0.0001, ~0.7<r<0.99) were observed between several metabolites and, in particular, for trioses (glyceraldehyde 3-phosphate, phosphoglycerate), purine metabolites (adenosine, adenosine monophosphate – AMP) and metabolites involved in glutathione homeostasis (5-oxoproline, glutamate – Figure 7).
Figure 7. Linear correlation of metabolite levels in AltitudeOmics RBCs and the concept of metabolic linkage.
Metabolite levels at each time point (sea level – SL, altitude 1, 7, 16 – ALT1, 7, 16, or following reascending 7 days after descending to 1525m – POST; color coded as detailed in the right hand panels) were correlated (Pearson linear correlation). Linear correlations (r) and statistical significance are provided for each panel. Metabolites showing linear correlations as high as ~0.9 are suggestive of the existence of a “metabolic linkage” between those metabolites, i.e. the relative levels of these metabolites are significantly dependent among each other. Sums were calculated by adding absolute values for linear correlations for each metabolite against other metabolites and physiological parameters. Results were thus sorted to obtain a rank of metabolites with the highest total correlations with other metabolites and physiological parameters, indicating their centrality in metabolic adaptations to hypoxia.
The bottom right panel summarizes the main metabolic adaptations observed in RBCs after acute and chronic exposure to high altitude hypoxia. Pathways are color-coded and arrow widths indicate relative fluxes through the pathway.
We thus calculated the sum of the absolute values of linear correlations for all metabolites and physiological values (Figure 7). This elaboration has been thought to suggest whether a set of metabolites with the highest total correlative values with other metabolites and physiological parameters might be regarded as key players in RBC metabolic adaptations to high altitude hypoxia. Results further indicated a preminent role for glyceraldehyde 3-phosphate, ribose phosphate and adenosine (Figure 7).
Discussion
The present study is part of the AltitudeOmics research program, a project that was designed to gain insights into adaptation to hypoxia and the retention of adaptation after return to low altitude through the study of physiological and metabolomics responses. This project involved twenty-one lowland volunteers in the field who were taken rapidly to 5260 m, where they acclimatized for 16 days. They then descended to 1525 m for 7 days, after which they returned quickly to 5260 m and were retested for physiological, behavioral, and physical parameters, as previously published.15,33–39 In parallel to these tests, RBC samples were collected at sea level (baseline), after 1, 7 and 16 days at high altitude, and following reascent after 7 days living at 1525m.
In the present study, we seek to investigate whether RBCs, key players in oxygen transport/delivery and a sink for the plasma metabolome, are metabolically influenced by exposure to high altitude hypoxia. This hypothesis was formulated in the light of in vitro evidence showing the presence of an oxygen-dependent metabolic modulation in RBCs. 26–30 Such adaptive mechanism results in the accumulation of NADPH for anti-oxidant purposes through the PPP under high oxygenation, since late glycolytic enzymes are sequestered (inhibitory binding) at the level of the cytosolic domain of band 3 and metabolic fluxes through the Embden-Meyerhof pathway are depressed. On the other hand, under low oxygenation, deoxyhemoglobin binding to the N-terminal cytosolic domain of band 3 displaces bound/inhibited glycolytic enzymes, thus promoting glycolysis and DPG generation, which in turn stabilizes the T state of hemoglobin. Ongoing glycolysis also promotes intracellular acidification. Increased proton availability thus favors the protonation of distal histidine and other key residues under deoxygenation,52 resulting in oxygen off-loading, a phenomenon referred to as the “Bohr effect”. While these metabolic adaptations have been consolidated through laboratory studies over the years, in vivo metabolomics evidence has not been generated yet, especially on a rare sample set such as lowlanders exposed to high altitude hypoxia. Mass spectrometry-based metabolomics is a useful tool to provide a broad overview of RBC metabolism under extreme conditions or various pathophysiological states, such as hereditary spherocytosis53 or sickle cell anemia45, in vitro aging of stored packed RBCs54–56 for transfusion purposes, and metabolic responses of RBCs to in vitro hypoxia31,32.
Here we confirmed for the first time that exposure to hypoxia results in an immediate enhancement of glycolysis and shut down of PPP in vivo in humans (a few hours after exposure on day 1), as evidenced by the significant and progressive accumulation of key triose phosphates and late glycolytic byproducts. However, while hypoxia is supposed to limit the antioxidant potential of RBCs in vitro30 and promote oxidative/reductive oxidative and nitrosative stress at high altitude,57 here we show that RBC metabolic adaptations to hypoxia in vivo result in higher levels of GSH and decreased GSSG. This finding is either suggestive of decreased oxidative stress or increased de novo biosynthesis of GSH, an ATP-dependent phenomenon58 that could be favored by transient increases of ATP levels during early responses observed on day 1 to 7, consistent with recent observations on anaerobically stored erythrocyte concentrates for transfusion purposes.59 This adaptation is notably lost at the POST time point, which showed a decrease in GSH levels and an increase in GSSG levels mirroring increased oxidative stress associated with transient re-exposure to normoxia.
Higher availability of ATP also resulted in the accumulation of AMP and adenosine, which in the light of the progressive accumulation of the non-oxidative phase PPP product ribose phosphate, is suggestive of ongoing phosphoribolysis. This effect is relevant in that circulating purines are known to stimulate coronary vasodilation60 in a nitric oxide-independent fashion,61 through targeting of specific receptors such as adenosine A(2B).62,63 Together with the accumulation of hypoxanthine, an adenine deamination byproduct that does not contribute to adenosine-induced coronary vasodilation64, these results are consistent with recent observations suggesting a mechanistic role for the purinergic system in driving RBC metabolic adaptations to hypoxia.63 Moreover, RBC xanthine oxidoreductase has been implicated in the erythrocytic activation of nitrite homeostasis65. Though follow-up targeted studies are mandatory to elucidate the regulatory mechanisms triggered by purine metabolites during adaptation to hypoxia, this hypothesis is further supported by correlative analyses showing a strong metabolic linkage between purine metabolites and triose phosphates generated by late glycolysis. In this view, it should be further noted that other purine analogues such as caffeine, a xanthine alkaloid, have been questioned to either have a beneficial or deleterious effect on adaptative responses (e.g. vasodilation).66
Nitric oxide-generating pathways (arginine catabolism and citrulline accumulation) were apparently upregulated upon early exposure to hypoxia, but were down-regulated after 7 days at high altitude, consistent with the hereby observed increase in asymmetric dimethyl-arginine, a nitric oxide synthase inhibitor.67 Of note, significant nitrite and nitrate increase were observed after 1 and 16 days at high altitude, and were preserved at the time of reascending one week after returning to 1525 m. Inorganic anions nitrate and nitrite were previously thought to be inert end products of endogenous nitric oxide (NO) metabolism.68 However, NO3− and NO2− can be recycled in vivo to form NO, a phenomenon mediated by xanthine oxidorectuase, ascorbate, polyphenols, myoglobins and protons or, in RBCs, by deoxyhemoglobin68. NO generation through reduction of nitrite by RBCs and deoxyhemoglobin promotes hypoxic vasodilation, inhibition of platelet reactivity through the activation of cGMP signaling.69–73,74 Our observations are thus consistent with the hypothesis that these anions might represent an important alternative source of NO to the classical L-arginine-NO-synthase pathway, in particular in hypoxic states.68
Arginine consumption could be also interpreted in the light of increases in creatine anabolism, especially after 7 and 16 days at high altitude. These results might indirectly mirror analogies between RBC metabolic adaptations to hypoxia and the role of the creatine pool and the quick availability of phosphocreatine in particular as a fast-mobilizing energy source for anaerobic activity in the muscle. In support of this finding, creatine supplementation has beneficial neuroprotective effects against transient cerebral hypoxia-ischemia in rats.75 These results are consistent with improved athletic performances of the AltitudeOmics subjects following 16 days at high altitude,33 even though it will be important to evaluate metabolic adaptations to high altitude hypoxia in relation to this pathway within the context of muscle metabolism.
Consumption of thiol/sulphur containing compounds cysteine, taurine and hypotaurine are here suggestive of alterations of sulphur metabolism during adaptation to hypoxia. In the absence of flux analyses, decreased levels of these compounds as seen here might either indicate increased consumption or decreased biosynthesis. An inhibitory effect of hydrogen sulfide (H2S) on hypoxia-inducible factor 1 (HIF1) in response to hypoxia has been previously reported.76 H2S is a RBC catabolic byproduct of sulphur-containing metabolites and a vasorelaxing molecule modulating vascular blood flow and pressure.51 Consistently, exposure to high altitude resulted in immediate decreases in H2S, an adaptation that was preserved after one week at 1525m.
Correlative analyses indicated a linkage between triose phosphates, pentose phosphate, sphingosine signaling and adaptive responses to hypoxia affecting physical performances, including gas transport (e.g. PaO2, PaCO2). Even though correlation does not imply causation, it is worthwhile to stress how metabolic reprogramming correlates with oxygen and CO2 homeostasis, two key variables mediating acclimatization to high altitude hypoxia. However, direct correlations with physical performance parameters >|0.5| were not observed. Of note, sphingosine kinase is a target of HIF signaling and participates in angiogenesis signaling to improve responses to hypoxia77,78. Overall, correlative results are indicative of “metabolic linkages” between pathways, such as glycolysis and salvage reactions. The current analysis also expands upon current knowledge of RBC metabolism, and the intertwinement of specific metabolic pathway adaptations within hours of exposure to hypoxia in vivo. Of note, these results inform about the preservation (“metabolic memory”) of such adaptations after one week from descent to lower altitudes, when other physiologic adaptations (higher hematocrit and hemoglobin levels12) are no longer retained, despite the persistence of measurable advantages in physical activity performances.33. In this view it is worth noting that also not all metabolic adaptations are retained after a second reascent, such as for example those related to the total levels of reduced glutathione, polyamines, creatine and carnitine metabolism. This is relevant in that it suggests that some of these metabolic adaptations observed during prolonged exposure to high altitude hypoxia may be a secondary effect of the main adaptations (e.g. glycolysis/PPP ratios, purine metabolism, nitrogen and hydrogen sulphide metabolism) that are actually needed to drive improved oxygen delivery and physical performances. Alternatively, some of these adapations may be necessary to compensate up/down-regulation of other pathways immediately after exposure to hypoxia(e.g. carnitine and creatine metabolism in energy/nitrogen metabolism), a mechanism that becomes unnecessary after acclimatization is established. Alternatively, this observation may rather indicate that prolonged and continuous stimuli are necessary to retain changes in those pathways that are restored to pre-ascent level upon transient descent to lower altitudes.
Conclusion
Here we applied metabolomics technologies to investigate the metabolic adaptation of human RBCs to high altitude hypoxia. The results impact the understanding of RBC responses to hypoxia in vivo, a basic biological question that expands beyond the scope of altitude research and into the fields of cardiovascular,1 polmunology2, trauma/hemorrhagic shock-induced hypoxemia4, and transfusion medicine3. We provide for the first time supportive evidence of RBC metabolic adaptations (bottom right panel - Figure 7) that ensue within hours from exposure to high altitude hypoxia.
Increases in glycolysis and deregulation of PPP was observed in RBCs from human volunteers ascending to 5260m, consistent with well-established in vitro models of oxygen-dependent metabolic modulation in human RBCs26–29. However, antioxidant potential in human RBCs was not limited by tuning down of PPP. Indeed, increased levels of the glutathione pool and decreased levels of precursor amino acids are suggestive of increased de novo synthesis of reducing equivalents. Arginine metabolism fueled the early accumulation of nitrite and nitrate (oxidation products of NO and a sink for NO generation under hypoxia). Arginine catabolism also corresponded to increases in the creatine pool, mirroring potential metabolic adaptations in muscles where increases in the creatine pool provide fast mobilizable energy sources to fuel physical activity under hypoxic conditions. Alterations to sulphur metabolism, as mirrored by altered levels of taurine, hypotaurine, cysteine and methionine, paralleled the observed adaptive deregulation of H2S in response to hypoxia. Finally, we provide the first in vivo evidence of the metabolic centrality of purines, triose and pentose phosphates, and sphingosine 1-phosphate in RBCs from volunteers exposed to high altitude hypoxia. We present correlative evidence between the levels of these metabolites and improved physiological parameters upon adaptation to hypoxia, such as gas transport, substantiating a role for metabolic modulation as an avenue to improve adaptive responses to hypobaric hypoxia.
Finally, we show that, contrary to other transient physiological adaptations (hematocrit and hemoglobin levels33), metabolic adaptations are retained after descending to lower altitude for one week, consistent with improved physical performance.
Supplementary Material
Supplementary Figure S1 Heat maps of Z-score normalized metabolomics analyses of RBCs at high altitude
Supplementary Figure S2 Hierarchical clustering analyses of correlation of metabolic parameters with metabolite levels and physiological readouts.
Metabolomics report
Statistical elaborations (ANOVA)
Correlation analysis and raw values of physiological data.
Acknowledgments
AD was supported by funds from the National Blood Foundation (Early career grant – 2016 cycle). The overall AltitudeOmics study was funded, in part, by grants from the United States Department of Defense (W81XWH-11-2-0040 TATRC to RCR and W81XWH-10-2-0114 to ATL). The project was also supported, in part, by National Institutes of Health (NIH)/National Center for Advancing Translational Sciences Colorado CTSI Grant Number UL1 TR000154. Contents are the author’s sole responsibility and do not necessarily represent official NIH views. Major additional support came from the Cardiopulmonary & Respiratory Physiology Laboratory, University of Oregon; the Altitude Research Center and the Charles S. Houston Endowed Professorship, Department of Emergency Medicine, School of Medicine, University of Colorado Denver. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest All the authors disclose no conflict of interests relevant to this study.
Explanation of Author Contributions RCR (AltitudeOmics), ADA and KCH (metabolomics) designed and supervised the overall experiment. RCR, AWS, ATL designed the study, collected the samples and monitored physiological parameters in loco during the expedition. ADA, TN, KCH set up the metabolomics platform, performed metabolomics analyses. KS, HL, AS, AAM, CGJ, KS, XY performed key adenosine assays and contributed to study design and interpretation. MTG, SS, GKK and CGK performed NO2-, NO3- or H2S measurements. ADA and DD performed statistical analyses. ADA and TN prepared figures and tables, and ADA wrote the paper. All authors critically commented on the manuscript.
References
- 1.Semenza GL. Hypoxia-Inducible Factor 1 and Cardiovascular Disease. Annu Rev Physiol. 2014;76(1):39–56. doi: 10.1146/annurev-physiol-021113-170322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stenmark KR, Fagan KA, Frid MG. Hypoxia-Induced Pulmonary Vascular Remodeling Cellular and Molecular Mechanisms. Circ Res. 2006;99(7):675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 3.D’Alessandro A, Kriebardis AG, Rinalducci S, Antonelou MH, Hansen KC, Papassideri IS, Zolla L. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2015;55(1):205–219. doi: 10.1111/trf.12804. [DOI] [PubMed] [Google Scholar]
- 4.Peltz E, D’Alessandro A, Moore E, Chin T, Silliman C, Sauaia A, Hansen K, Banerjee A. Pathologic metabolism: An exploratory study of the plasma metabolome of critical injury. J Trauma Acute Care Surg. 2015;78(4):742–51. doi: 10.1097/TA.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drew KL, Harris MB, LaManna JC, Smith MA, Zhu XW, Ma YL. Hypoxia tolerance in mammalian heterotherms. J Exp Biol. 2004;207(18):3155–3162. doi: 10.1242/jeb.01114. [DOI] [PubMed] [Google Scholar]
- 6.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83(4):1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- 7.Moore LG. Human genetic adaptation to high altitude. High Alt Med Biol. 2001;2(2):257–279. doi: 10.1089/152702901750265341. [DOI] [PubMed] [Google Scholar]
- 8.Duhm J, Gerlach E. On the mechanisms of the hypoxia-induced increase of 2,3-diphosphoglycerate in erythrocytes. Pflüg Arch. 1971;326(3):254–269. doi: 10.1007/BF00592506. [DOI] [PubMed] [Google Scholar]
- 9.Fencl V, Gabel RA, Wolfe D. Composition of cerebral fluids in goats adapted to high altitude. J Appl Physiol. 1979;47(3):508–513. doi: 10.1152/jappl.1979.47.3.508. [DOI] [PubMed] [Google Scholar]
- 10.Wood SC. Adaptation of Red Blood Cell Function to Hypoxia and Temperature in Ectothermic Vertebrates. Am Zool. 1980;20(1):163–172. [Google Scholar]
- 11.Samaja M, Brenna L, Allibardi S, Cerretelli P. Human red blood cell aging at 5,050-m altitude: a role during adaptation to hypoxia. J Appl Physiol Bethesda Md 1985. 1993;75(4):1696–1701. doi: 10.1152/jappl.1993.75.4.1696. [DOI] [PubMed] [Google Scholar]
- 12.Savourey G, Garcia N, Besnard Y, Hanniquet A-M, Fine M-O, Bittel J. Physiological changes induced by pre-adaptation to high altitude. Eur J Appl Physiol. 1994;69(3):221–227. doi: 10.1007/BF01094792. [DOI] [PubMed] [Google Scholar]
- 13.Savourey G, Garcia N, Caravel JP, Gharib C, Pouzeratte N, Martin S, Bittel J. Pre-adaptation, adaptation and de-adaptation to high altitude in humans: hormonal and biochemical changes at sea level. Eur J Appl Physiol. 1997;77(1–2):37–43. doi: 10.1007/s004210050297. [DOI] [PubMed] [Google Scholar]
- 14.Hornbein T, Schoene R. High Altitude: An Exploration of Human Adaptation. CRC Press; Washington, DC: 2001. pp. 1–986. [Google Scholar]
- 15.Ryan BJ, Wachsmuth NB, Schmidt WF, Byrnes WC, Julian CG, Lovering AT, Subudhi AW, Roach RC. AltitudeOmics: Rapid Hemoglobin Mass Alterations with Early Acclimatization to and De-Acclimatization from 5260 m in Healthy Humans. PloS One. 2014;9(10):e108788. doi: 10.1371/journal.pone.0108788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckardt KU, Boutellier U, Kurtz A, Schopen M, Koller EA, Bauer C. Rate of erythropoietin formation in humans in response to acute hypobaric hypoxia. J Appl Physiol Bethesda Md 1985. 1989;66(4):1785–1788. doi: 10.1152/jappl.1989.66.4.1785. [DOI] [PubMed] [Google Scholar]
- 17.Faura J, Ramos J, Reynafarje C, English E, Finne P, Finch CA. Effect of altitude on erythropoiesis. Blood. 1969;33(5):668–676. [PubMed] [Google Scholar]
- 18.Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16(10):1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- 19.Semenza GL. Regulation of Metabolism by Hypoxia-Inducible Factor 1. Cold Spring Harb Symp Quant Biol. 2011;76:347–353. doi: 10.1101/sqb.2011.76.010678. [DOI] [PubMed] [Google Scholar]
- 20.Ball MK, Waypa GB, Mungai PT, Nielsen JM, Czech L, Dudley VJ, Beussink L, Dettman RW, Berkelhamer SK, Steinhorn RH, et al. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1α. Am J Respir Crit Care Med. 2014;189(3):314–324. doi: 10.1164/rccm.201302-0302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenzo FR, Huff C, Myllymäki M, Olenchock B, Swierczek S, Tashi T, Gordeuk V, Wuren T, Ri-Li G, McClain DA, et al. A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet. 2014;46(9):951–956. doi: 10.1038/ng.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marín-Hernández A, Gallardo-Pérez JC, Ralph SJ, Rodríguez-Enríquez S, Moreno-Sánchez R. HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini Rev Med Chem. 2009;9(9):1084–1101. doi: 10.2174/138955709788922610. [DOI] [PubMed] [Google Scholar]
- 23.Tennant DA, Frezza C, MacKenzie ED, Nguyen QD, Zheng L, Selak MA, Roberts DL, Dive C, Watson DG, Aboagye EO, et al. Reactivating HIF prolyl hydroxylases under hypoxia results in metabolic catastrophe and cell death. Oncogene. 2009;28(45):4009–4021. doi: 10.1038/onc.2009.250. [DOI] [PubMed] [Google Scholar]
- 24.Monod J, Wyman J, Changeux J-P. On the nature of allosteric transitions: A plausible model. J Mol Biol. 1965;12(1):88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 25.D’Alessandro Angelo, Zolla Lello. Biochemistry of red cell aging in vivo and storage lesions. 2013. first. [Google Scholar]
- 26.Castagnola M, Messana I, Sanna MT, Giardina B. Oxygen-linked modulation of erythrocyte metabolism: state of the art. Blood Transfus. 2010;8(Suppl 3):s53–s58. doi: 10.2450/2010.009S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Low PS, Rathinavelu P, Harrison ML. Regulation of glycolysis via reversible enzyme binding to the membrane protein, band 3. J Biol Chem. 1993;268(20):14627–14631. [PubMed] [Google Scholar]
- 28.Giardina B, Messana I, Scatena R, Castagnola M. The Multiple Functions of Hemoglobin. Crit Rev Biochem Mol Biol. 1995;30(3):165–196. doi: 10.3109/10409239509085142. [DOI] [PubMed] [Google Scholar]
- 29.Messana I, Orlando M, Cassiano L, Pennacchietti L, Zuppi C, Castagnola M, Giardina B. Human erythrocyte metabolism is modulated by the O2-linked transition of hemoglobin. FEBS Lett. 1996;390(1):25–28. doi: 10.1016/0014-5793(96)00624-2. [DOI] [PubMed] [Google Scholar]
- 30.Rogers SC, Said A, Corcuera D, McLaughlin D, Kell P, Doctor A. Hypoxia limits antioxidant capacity in red blood cells by altering glycolytic pathway dominance. FASEB J. 2009;23(9):3159–3170. doi: 10.1096/fj.09-130666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yachie-Kinoshita A, Nishino T, Shimo H, Suematsu M, Tomita M. A metabolic model of human erythrocytes: practical application of the E-Cell Simulation Environment. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/642420. http://dx.doi.org/10.1155/2010/642420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Alessandro A, Gevi F, Zolla L. Red blood cell metabolism under prolonged anaerobic storage. Mol Biosyst. 2013;9(6):1196–1209. doi: 10.1039/c3mb25575a. [DOI] [PubMed] [Google Scholar]
- 33.Subudhi AW, Bourdillon N, Bucher J, Davis C, Elliott JE, Eutermoster M, Evero O, Fan J-L, Jameson-Van Houten S, Julian CG, et al. AltitudeOmics: the integrative physiology of human acclimatization to hypobaric hypoxia and its retention upon reascent. PloS One. 2014;9(3):e92191. doi: 10.1371/journal.pone.0092191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amann M, Goodall S, Twomey R, Subudhi AW, Lovering AT, Roach RC. AltitudeOmics: on the consequences of high-altitude acclimatization for the development of fatigue during locomotor exercise in humans. J Appl Physiol Bethesda Md 1985. 2013;115(5):634–642. doi: 10.1152/japplphysiol.00606.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan J-L, Subudhi AW, Evero O, Bourdillon N, Kayser B, Lovering AT, Roach RC. AltitudeOmics: enhanced cerebrovascular reactivity and ventilatory response to CO2 with high-altitude acclimatization and reexposure. J Appl Physiol Bethesda Md 1985. 2014;116(7):911–918. doi: 10.1152/japplphysiol.00704.2013. [DOI] [PubMed] [Google Scholar]
- 36.Goodall S, Twomey R, Amann M, Ross EZ, Lovering AT, Romer LM, Subudhi AW, Roach RC. AltitudeOmics: exercise-induced supraspinal fatigue is attenuated in healthy humans after acclimatization to high altitude. Acta Physiol Oxf Engl. 2014;210(4):875–888. doi: 10.1111/apha.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roach EB, Bleiberg J, Lathan CE, Wolpert L, Tsao JW, Roach RC. AltitudeOmics: Decreased reaction time after high altitude cognitive testing is a sensitive metric of hypoxic impairment. Neuroreport. 2014 doi: 10.1097/WNR.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subudhi AW, Fan J-L, Evero O, Bourdillon N, Kayser B, Julian CG, Lovering AT, Panerai RB, Roach RC. AltitudeOmics: cerebral autoregulation during ascent, acclimatization, and re-exposure to high altitude and its relation with acute mountain sickness. J Appl Physiol Bethesda Md 1985. 2014;116(7):724–729. doi: 10.1152/japplphysiol.00880.2013. [DOI] [PubMed] [Google Scholar]
- 39.Subudhi AW, Fan J-L, Evero O, Bourdillon N, Kayser B, Julian CG, Lovering AT, Roach RC. AltitudeOmics: effect of ascent and acclimatization to 5260 m on regional cerebral oxygen delivery. Exp Physiol. 2014;99(5):772–781. doi: 10.1113/expphysiol.2013.075184. [DOI] [PubMed] [Google Scholar]
- 40.D’Alessandro A, Nemkov T, Kelher M, West FB, Schwindt RK, Banerjee A, Moore EE, Silliman CC, Hansen KC. Routine storage of red blood cell (RBC) units in additive solution-3: a comprehensive investigation of the RBC metabolome. Transfusion. 2014;55(6):1155–68. doi: 10.1111/trf.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Alessandro A, Nemkov T, Hansen KC, Szczepiokowski ZM, Dumont LJ. Red blood cell storage in additive solution-7 preserves energy and redox metabolism: a metabolomics approach. Transfusion. 2015;55(12):2955–66. doi: 10.1111/trf.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clasquin MF, Melamud E, Rabinowitz JD. LC-MS data processing with MAVEN: a metabolomic analysis and visualization engine. Curr Protoc Bioinforma Ed Board Andreas Baxevanis Al. 2012;Chapter 14(Unit14.11) doi: 10.1002/0471250953.bi1411s37.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, Ognibene FP, Cannon RO. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci. 2000;97(21):11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peter EA, Shen X, Shah SH, Pardue S, Glawe JD, Zhang WW, Reddy P, Akkus NI, Varma J, Kevil CG. Plasma Free H2S Levels are Elevated in Patients With Cardiovascular Disease. J Am Heart Assoc. 2013;2(5):e000387. doi: 10.1161/JAHA.113.000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darghouth D, Koehl B, Junot C, Roméo P-H. Metabolomic analysis of normal and sickle cell erythrocytes. Transfus Clin Biol J Société Fr Transfus Sang. 2010;17(3):148–150. doi: 10.1016/j.tracli.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 46.Sana TR, Gordon DB, Fischer SM, Tichy SE, Kitagawa N, Lai C, Gosnell WL, Chang SP. Global mass spectrometry based metabolomics profiling of erythrocytes infected with Plasmodium falciparum. PloS One. 2013;8(4):e60840. doi: 10.1371/journal.pone.0060840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramírez-Zamora S, Méndez-Rodríguez ML, Olguín-Martínez M, Sánchez-Sevilla L, Quintana-Quintana M, García-García N, Hernández-Muñoz R. Increased erythrocytes byproducts of arginine catabolism are associated with hyperglycemia and could be involved in the pathogenesis of type 2 diabetes mellitus. PloS One. 2013;8(6):e66823. doi: 10.1371/journal.pone.0066823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleinbongard P, Schulz R, Rassaf T, Lauer T, Dejam A, Jax T, Kumara I, Gharini P, Kabanova S, Ozüyaman B, et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107(7):2943–2951. doi: 10.1182/blood-2005-10-3992. [DOI] [PubMed] [Google Scholar]
- 49.Yang J, Gonon AT, Sjöquist P-O, Lundberg JO, Pernow J. Arginase regulates red blood cell nitric oxide synthase and export of cardioprotective nitric oxide bioactivity. Proc Natl Acad Sci U S A. 2013;110(37):15049–15054. doi: 10.1073/pnas.1307058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krotova K, Patel JM, Block ER, Zharikov S. Hypoxic upregulation of arginase II in human lung endothelial cells. Am J Physiol Cell Physiol. 2010;299(6):C1541–C1548. doi: 10.1152/ajpcell.00068.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner CA. Hydrogen sulfide: a new gaseous signal molecule and blood pressure regulator. J Nephrol. 2009;22(2):173–176. [PubMed] [Google Scholar]
- 52.Kovalevsky A, Chatake T, Shibayama N, Park S-Y, Ishikawa T, Mustyakimov M, Fisher SZ, Langan P, Morimoto Y. Protonation states of histidine and other key residues in deoxy normal human adult hemoglobin by neutron protein crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 11):1144–1152. doi: 10.1107/S0907444910025448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darghouth D, Koehl B, Heilier JF, Madalinski G, Bovee P, Bosman G, Delaunay J, Junot C, Roméo P-H. Alterations of red blood cell metabolome in overhydrated hereditary stomatocytosis. Haematologica. 2011;96(12):1861–1865. doi: 10.3324/haematol.2011.045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97(1):107–115. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Amici GM, Mirasole C, D’Alessandro A, Yoshida T, Dumont LJ, Zolla L. Red blood cell storage in SAGM and AS3: a comparison through the membrane two-dimensional electrophoresis proteome. Blood Transfus. 2012;10(Suppl 2):s46–s54. doi: 10.2450/2012.008S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D’Alessandro A, Hansen KC, Silliman CC, Moore EE, Kelher M, Banerjee A. Metabolomics of AS-5 RBC supernatants following routine storage. Vox Sang. 2014;108(2):131–40. doi: 10.1111/vox.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dosek A, Ohno H, Acs Z, Taylor AW, Radak Z. High altitude and oxidative stress. Respir Physiol Neurobiol. 2007;158(2–3):128–131. doi: 10.1016/j.resp.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 58.Raftos JE, Whillier S, Kuchel PW. Glutathione Synthesis and Turnover in the Human Erythrocyte Alignment of a model based on detailed enzyme kinetics with experimental data. J Biol Chem. 2010;285(31):23557–23567. doi: 10.1074/jbc.M109.067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reisz JA, Wither MJ, Dzieciatkowska M, Nemkov T, Issaian A, Yoshida T, Dunham AJ, Hill RC, Hansen KC, D’Alessandro A. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016 doi: 10.1182/blood-2016-05-714816. [DOI] [PubMed] [Google Scholar]
- 60.Sato A, Terata K, Miura H, Toyama K, Loberiza FR, Hatoum OA, Saito T, Sakuma I, Gutterman DD. Mechanism of vasodilation to adenosine in coronary arterioles from patients with heart disease. Am J Physiol - Heart Circ Physiol. 2005;288(4):H1633–H1640. doi: 10.1152/ajpheart.00575.2004. [DOI] [PubMed] [Google Scholar]
- 61.Costa F, Biaggioni I. Role of Nitric Oxide in Adenosine-Induced Vasodilation in Humans. Hypertension. 1998;31(5):1061–1064. doi: 10.1161/01.hyp.31.5.1061. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y, Dai Y, Wen J, Zhang W, Grenz A, Sun H, Tao L, Lu G, Alexander DC, Milburn MV, et al. Detrimental effects of adenosine signaling in sickle cell disease. Nat Med. 2011;17(1):79–86. doi: 10.1038/nm.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu H, Zhang Y, Wu H, D’Alessandro A, Yegutkin GG, Song A, Sun K, Li J, Cheng N-Y, Huang A, et al. Beneficial Role of Erythrocyte Adenosine A2B Receptor–Mediated AMP-Activated Protein Kinase Activation in High-Altitude HypoxiaClinical Perspective. Circulation. 2016;134(5):405–421. doi: 10.1161/CIRCULATIONAHA.116.021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saito D, Mima T, Obayashi N, Uchida S, Maekawa K, Sato T, Mizuo K, Kobayashi H, Haraoka S. Effects of inosine on adenosine-induced coronary vasodilation in the open chest dog. Arzneimittelforschung. 1993;43(9):950–953. [PubMed] [Google Scholar]
- 65.Liu C, Wajih N, Liu X, Basu S, Janes J, Marvel M, Keggi C, Helms CC, Lee AN, Belanger AM, et al. Mechanisms of human erythrocytic bioactivation of nitrite. J Biol Chem. 2015;290(2):1281–1294. doi: 10.1074/jbc.M114.609222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hackett PH. Caffeine at high altitude: java at base cAMP. High Alt Med Biol. 2010;11(1):13–17. doi: 10.1089/ham.2009.1077. [DOI] [PubMed] [Google Scholar]
- 67.Billecke SS, Kitzmiller LA, Northrup JJ, Whitesall SE, Kimoto M, Hinz AV, D’Alecy LG. Contribution of whole blood to the control of plasma asymmetrical dimethylarginine. Am J Physiol Heart Circ Physiol. 2006;291(4):H1788–H1796. doi: 10.1152/ajpheart.00066.2006. [DOI] [PubMed] [Google Scholar]
- 68.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 69.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 70.Akrawinthawong K, Park JW, Piknova B, Sibmooh N, Fucharoen S, Schechter AN. A flow cytometric analysis of the inhibition of platelet reactivity due to nitrite reduction by deoxygenated erythrocytes. PloS One. 2014;9(3):e92435. doi: 10.1371/journal.pone.0092435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Velmurugan S, Kapil V, Ghosh SM, Davies S, McKnight A, Aboud Z, Khambata RS, Webb AJ, Poole A, Ahluwalia A. Antiplatelet effects of dietary nitrate in healthy volunteers: involvement of cGMP and influence of sex. Free Radic Biol Med. 2013;65:1521–1532. doi: 10.1016/j.freeradbiomed.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park JW, Piknova B, Huang PL, Noguchi CT, Schechter AN. Effect of blood nitrite and nitrate levels on murine platelet function. PloS One. 2013;8(2):e55699. doi: 10.1371/journal.pone.0055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corti P, Tejero J, Gladwin MT. Evidence mounts that red cells and deoxyhemoglobin can reduce nitrite to bioactive NO to mediate intravascular endocrine NO signaling: commentary on “Anti-platelet effects of dietary nitrate in healthy volunteers: involvement of cGMP and influence of sex”. Free Radic Biol Med. 2013;65:1518–1520. doi: 10.1016/j.freeradbiomed.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 74.Gladwin MT, Wang X, Reiter CD, Yang BK, Vivas EX, Bonaventura C, Schechter AN. S-Nitrosohemoglobin is unstable in the reductive erythrocyte environment and lacks O2/NO-linked allosteric function. J Biol Chem. 2002;277(31):27818–27828. doi: 10.1074/jbc.M203236200. [DOI] [PubMed] [Google Scholar]
- 75.Adcock KH, Nedelcu J, Loenneker T, Martin E, Wallimann T, Wagner BP. Neuroprotection of creatine supplementation in neonatal rats with transient cerebral hypoxia-ischemia. Dev Neurosci. 2002;24(5):382–388. doi: 10.1159/000069043. [DOI] [PubMed] [Google Scholar]
- 76.Kai S, Tanaka T, Daijo H, Harada H, Kishimoto S, Suzuki K, Takabuchi S, Takenaga K, Fukuda K, Hirota K. Hydrogen sulfide inhibits hypoxia- but not anoxia-induced hypoxia-inducible factor 1 activation in a von hippel-lindau- and mitochondria-dependent manner. Antioxid Redox Signal. 2012;16(3):203–216. doi: 10.1089/ars.2011.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cuvillier O, Ader I. Hypoxia-inducible factors and sphingosine 1-phosphate signaling. Anticancer Agents Med Chem. 2011;11(9):854–862. doi: 10.2174/187152011797655050. [DOI] [PubMed] [Google Scholar]
- 78.Sun K, Zhang Y, D’Alessandro A, Nemkov T, Song A, Wu H, Liu H, Adebiyi M, Huang A, Wen YE, et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat Commun. 2016;7:12086. doi: 10.1038/ncomms12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 Heat maps of Z-score normalized metabolomics analyses of RBCs at high altitude
Supplementary Figure S2 Hierarchical clustering analyses of correlation of metabolic parameters with metabolite levels and physiological readouts.
Metabolomics report
Statistical elaborations (ANOVA)
Correlation analysis and raw values of physiological data.