Abstract
Purpose
To validate the Ocular Pain Assessment Survey (OPAS), specifically designed to measure ocular pain and quality of life for use by eye care practitioners and researchers.
Design
A single-center, cohort study was conducted in patients with and without corneal and ocular surface pain at initial and follow-up visits over a 6-month period. The content of the OPAS was guided by literature review, a body of experts, and incorporating conceptual frameworks from existing pain questionnaires. The Wong-Baker FACES® Pain Rating Scale served as the gold standard for measuring intensity of ocular pain.
Subjects
102 patients aged 18 to 80 years completed the OPAS at the initial visit. 21 patients were followed up post-treatment.
Methods and Statistical Analysis
Indices of validity and internal consistency (Spearman’s rank-order, rs, or Pearson’s correlation coefficients, rp), and coefficient of reliability (Cronbach’s α) were determined in addition to equivalence testing, exploratory factor analysis (EFA) and diagnostic analysis.
Main outcome measures
Eye pain intensity was the primary outcome measure, while interference with quality of life (QoL), aggravating factors, associated factors, associated non-eye pain intensity and self-reported symptomatic relief were the secondary outcome measures.
Results
The OPAS had criterion validity at both initial (rs= 0.71, n= 102, P<0.01) and follow-up visits (rs= 0.97, n= 21, P<0.01). Equivalence tests yielded OPAS and gold standard equivalence for both the initial and follow-up visits. EFA supported 6 sub-scales (eye pain intensity 24 h and 2 weeks, non-eye pain intensity, quality of life, aggravating factors, and associated factors) confirming multi-dimensionality. Cronbach’s α >0.83 for all sub-scales established strong internal consistency, which correlated with the gold standard, including 24-hour eye pain intensity and QoL interference scores (rp = 0.81, 0.64, P< 0.001). At follow-up, reduction in pain scores was accompanied by improvement in all dimensions of the OPAS. Percent change in QoL correlated to percent change in the gold standard (rp = 0.53, P<0.05). The OPAS was sensitive (94%), specific (81%) and accurate (91%) with a diagnostic odds ratio greater than 50.
Conclusions
The OPAS is a valid, reliable and responsive tool with strong psychometric and diagnostic properties in the multi-dimensional quantification of corneal and ocular surface pain intensity, and quality of life.
Ocular pain is one of the chief complaints for which patients seek ophthalmic medical help.1–3 Ocular pain may be due to various etiologies ranging from infection, inflammation or trauma at the ocular surface, neuropathic pain, or inflammation of the posterior segment. The cornea is the most densely innervated organ of the body with 7,000 nociceptors/mm2.4 These corneal nerves are vulnerable to damage from infections, inflammation, surgery, trauma, radiation and medications, both local and systemic. Ocular pain is associated with a significant decline in the quality of life and patients with corneal pain may suffer from anxiety, depression and suicidal ideation.5 Pharmacologic management of ocular pain and development of new therapeutic modalities are limited in part by a reliable and validated metric to measure ocular pain. Given the strong interest in spearheading the development of biologics to effectively reduce ocular pain, there is an eminent need for reliable metrics that can aid the physician in making an objective and accurate assessment of pain at baseline, and the therapeutic response both in terms of pain intensity and its impact on activities of daily life.6 While several validated questionnaires exist for quantification of symptom severity, visual function, and quality of life in patients with dry eye disease,7 such as the McMonnies dry eye questionnaire,8, 9 Ocular Surface Disease Index (OSDI) questionnaire,10 Standardized Patient Evaluation for Eye Dryness (SPEED) questionnaire,11 Symptom Assessment in Dry Eye (SANDE) questionnaire,7,12,13 and the National Eye Institute Vision Function Questionnaire (NEI-VFQ),14,15 a quantitative, validated tool tailored to measuring ocular pain has yet to be developed. Currently, ophthalmologists and optometrists use general pain scales such as visual analog scales (VAS), numerical rating scales (NRS) and categorical scales that employ verbal descriptors or sub-scales from the above mentioned questionnaires.16–19 The Wong-Baker FACES® Pain Rating Scale, which was developed in the 1980s, has been used extensively in pain research and clinical practice including clinical trials.20–26 To date, there is one available method for specifically evaluating ocular discomfort: the Eye Sensation Scale.27 The Eye Sensation Scale is a validated questionnaire described by Caudle and colleagues in 2007 that grades sensations of ocular discomfort, not necessarily pain, using a 5-category descriptive scale (excruciating, severe, moderate, mild, none),27 therefore it is not specific to ocular pain and does not provide a quantitative score that may be monitored.
In 2010, the National Eye Institute held a workshop on ocular pain and sensitivity, which concluded that there was need for a validated tool that could measure and quantify pain.5 The workshop also emphasized that current eye pain questionnaires were yet to be validated.5 Furthermore, clinical trials in eye pain also necessitate validated outcome measures for eye pain. Given this demand among eye care practitioners, scientists and industry, we sought to develop and test the psychometric properties of a novel ocular pain questionnaire with numerical and quantifiable rating scales, the Ocular Pain Assessment Survey (OPAS), specifically designed to measure ocular pain intensity in patients with eye pain of any origin. In establishing criterion-related validity, a gold standard is chosen that is the currently best available diagnostic test or benchmark of the condition, which is why The Wong-Baker FACES® Pain Rating Scale was chosen. Moreover, we added dimensionality to the questionnaire by factoring in questions regarding quality of life (QoL), aggravating factors, associated factors and non-eye related pain, yielding a score for each dimension of the OPAS that may be tracked over the course of treatment. We demonstrate that in our study population comprising patients with and without corneal and ocular surface pain, the OPAS is a valid, reliable, internally consistent and responsive tool in quantifying and monitoring ocular pain intensity. The ocular pain intensity test scales are sensitive and specific for ocular pain with strong predictive values and high accuracy in correctly detecting ocular pain. The utility of the OPAS is bolstered by its multi-dimensionality, which offers quantitative assessment and tracking of other ocular pain-related parameters of possible interest, such as QoL, aggravating factors, associated factors and non-ocular pain.
METHODS
Study Design and Subjects
We conducted a prospective, single-center, study to test and validate the OPAS among patients presenting to the Cornea Service at the Massachusetts Eye and Ear Infirmary (MEEI), Boston, MA over a period of 6 months (2011) (Figure 1), with exemption provided by the Institutional Review Board (IRB) at MEEI. All methods described in our study adhered to the tenets of the Declaration of Helsinki and were in compliance with the Health Insurance Portability and Accountability Act (HIPAA). Based on IRB exemption, subjects completing the questionnaire were not required to provide informed consent to their participation and use of data, including publication of unidentifiable data. The sample size calculation showed that 90 subjects would provide 85% power with a null hypothesis correlation of 0.70, an alternative hypothesis correlation of 0.82, and a one-sided 5% alpha.
Figure 1. Study design algorithm.
Flow of the steps taken toward designing and validating the Ocular Pain Assessment Survey (OPAS). MEEI: Massachusetts Eye and Ear Infirmary, Boston, MA, USA.
Over a course of 6 months, the OPAS was distributed to 288 English-speaking patients presenting to the Cornea Service (MEEI) both with and without complaints of ocular surface pain, aged 18–80 years, regardless of sex and race. Patients used the self-administration technique to respond to the questionnaire. 56 patients also completed the OPAS at their follow-up visits. Only patients who responded to the gold standard pain rating question (Qs. 1) in addition to the test questions of the OPAS were included in the study, and those who failed to respond to Qs. 1 were excluded. Consequently, 102 consecutive patients were included in the study, of which 21 also had a follow-up visit post-treatment. The remaining patients had failed to respond to the gold standard pain rating scale (Qs. 1), meeting the exclusion criterion for our validation studies. Following validation studies and factor analysis, the OPAS received minor revision in format while retaining questions in their original wording to generate its final version.
Questionnaire Design
We designed an initial 32-question, 8-domain, ocular pain questionnaire using numerical rating scales to assess eye pain intensity (worse eye) in the past 24 hours and 2 weeks (worst, least and average pain intensity), frequency of eye and non-eye pain (past 24 hours and 2 weeks), non-eye pain intensity (past 24 hours and 2 weeks), impact on QoL, pre-occupation with eye and non-eye pain, aggravating factors, associated factors and symptomatic relief (Supplementary Figure 1; available at http://www.aao.journal.org). Questions on frequency of pain were included to allow assessment of discriminant validity of the OPAS since hypothetically, intensity of ocular pain should not demonstrate any observable relationship with the frequency of ocular pain. Each test question was scored on a scale of 0–10, or 0–100, with increments of 1 or 10 units, respectively.
The design of the OPAS drew upon relevant features of some of the currently available validated systemic pain questionnaires, such as the McGill pain questionnaire, Brief Pain Inventory (BPI), and a local qualitative eye pain questionnaire, the Boston Foundation for Sight (BFS) Eye Pain Index (not validated), further incorporating ocular pain-specific questions recommended by a body of expert ophthalmologists (PH, RD). For the purpose of validation and reliability studies, we incorporated the established and widely used Wong-Baker FACES® Pain Rating Scale,21–26 as a gold standard method for measuring ocular pain intensity.
Statistical Analysis
All statistical analyses were performed using the Statistical Analysis Software (SAS version 9.4, North Carolina, USA).
(a) Reliability and Internal Consistency
Reliability and internal consistency of the OPAS were assessed using Cronbach’s α and Spearman’s rank (rs) or Pearson’s (rp) correlation coefficients.
(b) Validity and Exploratory Factor Analysis
Face validity was determined by the body of ophthalmologists, research personnel and statistician developing the OPAS; response rates for all test questions were reviewed to determine any poorly designed questions and provide a measure of linguistic validity28; criterion-related (external) validity of the OPAS was assessed by equivalence testing with an equivalence bound of ±0.75 against the gold standard pain intensity scale score, the Wong-Baker FACES® Pain Rating Scale, and correlations (rs, rp) between the gold-standard Wong-Baker FACES® Pain Rating Scale scores and test question scores from the initial visit.
An exploratory factor analysis (EFA) was performed to determine the factors (dimensions) being measured by the OPAS and to identify any questions that would not load well onto any factors within the OPAS. Questions that loaded highly onto the same factors were aggregated and a mean composite score was generated for each of those factors (dimensions) for further analysis (Supplementary Figure 2; available at http://www.aao.journal.org).
(c) Responsiveness and Longitudinal Validity
Post-treatment follow-up composite scores for each dimension were used to establish responsiveness and longitudinal validity of the OPAS, both of which are critical properties of a questionnaire in clinical trials. Composite scores for each dimension of the OPAS were calculated as the mean score of its constituent scales (Supplementary Figure 2; available at http://www.aao.journal.org). Responsiveness was measured by the change in mean (composite) scores between the initial and follow-up visits using the gold standard Wong-Baker FACES® Pain Rating Scale and each dimension of the OPAS. Longitudinal validity was measured by the strength of correlation of changes in pain intensity scores between the gold standard Wong-Baker FACES® Pain Rating Scale and the 24-hour ocular pain intensity scale.
(a) Diagnostic Analysis
Detailed diagnostic analysis of the 24-hour ocular pain factor was established by assessing its sensitivity, specificity, accuracy, predictive values and likelihood ratios. Data were dichotomized into the presence or absence of ocular pain using the gold standard Wong-Baker FACES® Pain Rating Scale (0 = pain absent, >0 = pain present),29 and the composite 24-hour ocular pain intensity scores. Responses to the gold standard Wong-Baker FACES® Pain Rating Scale were used to calculate prevalence of ocular pain in this study cohort.
RESULTS
Demographics
288 subjects completed at least one item of the OPAS, of which 102 subjects (mean age 48.9±15.7 years, age range 18–80 years) were included in the study at the initial visit based on meeting the minimum requirement of filling both the gold standard pain scale (Qs. 1) and at least one of the test pain scales (Qs. 5–10). 56 subjects also had a follow-up visit, at which time the OPAS was self-administered again; 21 subjects (mean age 46.1±17.2 years, age range 23–80 years) successfully met the criteria for inclusion. The etiology of corneal and ocular surface pain included infectious and non-infectious keratitis, corneal ulcers, dry eye disease, ocular graft-versus-host disease, allergic conjunctivitis, keratoconus and refractive surgery. The pain intensity scores among patients with eye pain at the initial visit as measured by the gold standard and 24hour eye pain intensity test scale (composite score) were 3.48±2.27 (n=81) and 3.23±2.05 (n=80, P=0.48), respectively.
Face Validity and Exploratory Factor Analysis
The group of ophthalmologists, research personnel and study statistician determined that the OPAS had face validity considering the layout, and structure of the OPAS. Based on the response rates for each question, questions of poor design with lower response rates, and consequently poor linguistic validity, were eliminated from the final questionnaire (Supplementary Tables 1 and 2; available at http://www.aao.journal.org). Therefore, the 4 questions on the frequency of pain were removed from the final questionnaire. Response rates were high (81.4% to 100%) for all questions of the OPAS covering eye and non-eye pain intensity, quality of life, aggravating factors, associated symptoms and preoccupation with pain (Supplementary Tables 1 and 2; available at http://www.aao.journal.org) suggesting that components of the OPAS were linguistically valid. From among the questions regarding self-reported symptomatic relief at the follow-up visit, the question on ocular pain had a better response rate (90.5%) than its non-ocular pain counterpart (61.9% of subjects responded to this question) (Supplementary Table 2; available at http://www.aao.journal.org).
EFA identified that indeed the OPAS was multi-dimensional and grouped the questions into 6 distinct factors (dimensions, Figure 2). An additional factor emerged that grouped all non-eye pain related questions under one factor. Furthermore, EFA revealed that the 2 questions on symptom relief did not load highly onto any of the identified factors. The authors decided that these two questions (Qs. 27 and 28, Figure 2) would be retained since they provided useful patient self-reported information that could be analyzed separately as needed, in particular for follow-up visits. Hence, the revised OPAS comprised of 6 identified factors making it a multidimensional assessment tool: 1. Eye pain intensity in the past 24 hours, 2. Eye pain intensity in the past 2 weeks, 3. Non-eye pain questions, 4. QoL, 5. Aggravating factors, and 6. Associated symptoms. Each factor generated a composite score based on the mean of its constituent scale scores as shown in (Supplementary Figure 2; available at http://www.aao.journal.org). Therefore, each patient had 6 scores, one for each factor, which may be tracked over time.
Figure 2. Final design of the Ocular Pain Assessment Survey (OPAS) following factor analysis and validation studies.
The boxed regions represent the gold standard method of quantifying pain intensity (Wong-Baker FACES Pain Rating Scale) against which the test numerical rating scales were validated, and the 6 factors (dimensions) of the OPAS comprising of test sub-scales that loaded onto each of the factors. Scores for each of the sub-scales were then followed as mean composite scores for each of the 6 factors, and monitored individually over time, generating both holistic and nuanced information. Symptomatic relief (Qs. 27 and 28) did not load onto any factors but was retained based on the physicians’ agreement that it formed an important question, which could be analyzed separately if needed.
Criterion Validity and Equivalence Test
Criterion-Related Validity
The Wong-Baker FACES® Pain Rating Scale (gold standard pain intensity scale) used to measure eye pain intensity showed good to very good correlation with all test ocular pain intensity questions at both initial and follow-up visits, demonstrating criterion validity of these pain intensity questions (Table 1). Furthermore, the observed correlation between the 24 hour ocular pain intensity dimension and the gold standard was r= 0.81 (Table 2), significantly greater than the null hypothesis correlation of r= 0.70 in the design (P= 0.01).
Table 1.
Criterion validity of ocular pain intensity questions of the Ocular Pain Assessment Survey (OPAS).
| INITIAL VISIT | ||||||
|---|---|---|---|---|---|---|
| Most eye pain intensity in the past 24h |
Most eye pain intensity in the past 2 weeks |
Least eye pain intensity in the past 24h |
Least eye pain intensity in the past 2 weeks |
Average eye pain intensity in the past 24h |
Average eye pain intensity in the past 2 weeks |
|
|
GOLD STD (pain intensity) |
0.71 | 0.51 | 0.70 | 0.46 | 0.72 | 0.53 |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| N | 101 | 95 | 102 | 93 | 102 | 91 |
| FOLLOW-UP VISIT | ||||||
|---|---|---|---|---|---|---|
| Most eye pain intensity in the past 24h |
Most eye pain intensity in the past 2 weeks |
Least eye pain intensity in the past 24h |
Least eye pain intensity in the past 2 weeks |
Average eye pain intensity in the past 24h |
Average eye pain intensity in the past 2 weeks |
|
|
GOLD STD (pain intensity) |
0.70 | 0.76 | 0.91 | 0.84 | 0.97 | 0.79 |
| P | 0.0004 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| N | 21 | 20 | 20 | 19 | 21 | 20 |
Criterion validity was determined by the strength of correlation (Spearman’s rank correlation coefficient, rs) between the gold standard (gold std) pain intensity scale, the Wong-Baker FACES® Pain Rating Scale, and all constituent scales of the ocular pain intensity questions. All ocular pain intensity questions of the OPAS demonstrated strong criterion validity, which was maintained at the follow-up visit as well. The total number of subjects enrolled at the initial and follow-up visits were 102 and 21, respectively. A p-value of less than 0.05 was considered statistically significant.
Table 2.
Criterion (discriminant) validity of non-pain dimensions of the Ocular Pain Assessment Survey (OPAS).
| QoL QUESTIONS | |||||||
|---|---|---|---|---|---|---|---|
| Reading and computer use |
Driving and watching TV |
General activity |
Mood | Sleep | Relationships in life |
Thinking about eye pain |
|
|
GOLD STD (pain intensity) |
0.51 | 0.59 | 0.64 | 0.67 | 0.46 | 0.59 | 0.61 |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| N | 89 | 86 | 83 | 86 | 85 | 87 | 91 |
| AGGRAVATING FACTORS | ASSOCIATED SYMPTOMS | SYMPTOM RELIEF | ||||||
|---|---|---|---|---|---|---|---|---|
| Wind/ Dry air/ Air con |
Volatile chemicals |
Redness | Burning | Sensitivity to light |
Tearing | Eye pain | Non-eye pain |
|
|
GOLD STD (pain intensity) |
0.48 | 0.29 | 0.19 | 0.37 | 0.37 | 0.25 | −0.72 | −0.48 |
| P | <0.0001 | 0.007 | 0.064 | 0.0004 | 0.0004 | 0.019 | 0.0005 | 0.09 |
| N | 94 | 86 | 89 | 86 | 88 | 88 | 19 | 13 |
Criterion validity was determined by the strength of correlation (Spearman’s rank correlation coefficient, rs) between the gold standard (gold std) pain intensity scale, the Wong-Baker FACES® Pain Rating Scale, and all constituent questions of the non-pain intensity dimensions of the OPAS encompassing interference with the quality of life (QoL), aggravating factors, associated symptoms, and symptomatic relief. As expected and in support of discriminant validity of the OPAS, non-pain intensity questions had lower correlations with the gold standard pain intensity scale as compared to pain intensity scales (Table 1), suggesting that while these questions did not measure pain intensity, they were related to ocular pain. The total number of subjects enrolled at the initial and follow-up visits were 102 and 21, respectively. All dimensions were assessed using data from the initial visit except for symptomatic relief, for which data were used from the follow-up visit. A p-value of less than 0.05 was considered statistically significant.
Non-ocular pain intensity questions had lower correlations (rs= 0.37, 0.48, P<0.001) in support of the premise that the non-ocular pain questions in fact do measure pain of another origin.
Discriminant Validity
The OPAS exhibited discriminant validity (Table 2) by the lesser correlation between the Wong-Baker FACES® Pain Rating Scale (gold standard pain intensity scale) and test questions for both ocular and non-ocular pain. Questions under all other dimensions of the OPAS showed mild to good criterion validity (Table 2) confirming that while the dimensions were not direct measures of ocular pain intensity, they were related to ocular pain intensity to varying degrees. This further supported discriminant validity of the OPAS through lower correlation coefficients for non-pain intensity questions (Table 2) as compared to pain intensity-specific questions (Table 1).
In addition to scores from individual questions, composite scores from each of the 6 dimensions of the OPAS also yielded similar results for convergent and discriminant validity (Table 3).
Table 3.
Criterion validity using composite scores of all dimensions of the Ocular Pain Assessment Survey (OPAS).
| DIMENSIONS OF THE OPAS – INITIAL VISIT | ||||||
|---|---|---|---|---|---|---|
| Eye pain intensity in the past 24h |
Eye pain intensity in the past 2 weeks |
QoL | Aggravating factors |
Associated symptoms |
Non-eye pain intensity |
|
|
GOLD STD (pain intensity) |
0.81 | 0.57 | 0.64 | 0.41 | 0.33 | 0.47 |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.01 | <0.0001 |
| N | 102 | 95 | 102 | 94 | 92 | 102 |
| DIMENSIONS OF THE OPAS – FOLLOW-UP VISIT | ||||||
|
GOLD STD (pain intensity) |
0.93 | 0.68 | 0.74 | 0.64 | 0.33 | 0.19 |
| P | <0.0001 | <0.001 | <0.001 | <0.01 | >0.05 | >0.05 |
| N | 21 | 18 | 21 | 21 | 19 | 21 |
Composite scores from each of the 6 dimensions of the OPAS were correlated (Spearman’s correlation coefficient, rs) with scores from the gold standard Wong-Baker FACES Pain Rating Scale (gold std) at initial (n=102) and follow-up visits (n=21). Ocular pain intensity and interference with quality of life (QoL) dimensions showed the strongest correlations, and lower but significant correlations for aggravating factors, associated factors and non-eye pain intensity dimensions. These results confirm both convergent and discriminant validity of the OPAS that was consistent at the follow-up visit as well. A p-value of less than 0.05 was considered statistically significant.
Equivalence testing
Equivalence testing with an equivalence bound of ±0.75 enabled us to conclude that the gold standard pain intensity and the test 24-hour average ocular pain intensity scores were equivalent at both the initial (95% CI around the mean difference −0.06, 0.65) and follow-up visits (95% CI around the mean difference −0.49, 0.06).
Reliability and Internal Consistency
All dimensions of the OPAS had good to excellent reliability (Table 4) with Cronbach’s α greater than or equal to 0.83. Questions within the ocular pain intensity and QoL dimensions displayed strong internal consistency, with good to excellent correlations between scores of the questions and the mean (composite) score for the dimension (Table 5). Likewise, the other dimensions of the OPAS: aggravating factors, associated symptoms and non-ocular pain, were also internally consistent with correlation coefficients (rp ) ranging from 0.92–0.94, 0.80–0.84 and 0.57–0.90 (all P<0.0001), respectively.
Table 4.
Reliability of the Ocular Pain Assessment Survey (OPAS).
| Dimension of the OPAS | Cronbach’s α |
|---|---|
| Ocular pain intensity (past 24h) | 0.88 |
| Ocular pain intensity (past 2 weeks) | 0.91 |
| Interference with quality of life | 0.95 |
| Aggravating factors | 0.84 |
| Associated symptoms | 0.83 |
| Non-ocular pain | 0.84 |
All dimensions of the OPAS had good to excellent reliability with Cronbach’s α ranging from 0.83 to 0.95.
Table 5.
Internal consistency of the Ocular Pain Assessment Survey (OPAS).
| OCULAR PAIN INTENSITY QUESTIONS | |||||||
|---|---|---|---|---|---|---|---|
|
Most eye pain intensity in the past 24h |
Least eye pain intensity in the past 24h |
Average eye pain intensity in the past 24h |
2-week intensity score P N |
Most eye pain intensity in the past 2 weeks |
Least eye pain intensity in the past 2 weeks |
Average eye pain intensity in the past 2 weeks |
|
|
24h intensity score |
0.92 | 0.86 | 0.92 | 0.93 | 0.87 | 0.96 | |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.001 | |
| N | 101 | 102 | 102 | 95 | 93 | 91 | |
| QUALITY OF LIFE QUESTIONS | |||||||
|---|---|---|---|---|---|---|---|
| Reading and computer use |
Driving and watching TV |
General activity |
Mood | Sleep | Relationships in life |
Thinking about eye pain |
|
| QoL score | 0.69 | 0.74 | 0.81 | 0.83 | 0.70 | 0.80 | 0.69 |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| N | 89 | 86 | 83 | 86 | 85 | 87 | 91 |
Internal consistency was determined by the strength of correlation (Pearson’s correlation coefficient, rp) between each dimension’s composite score and individual constituent questions of that dimension. Data from the initial visit were used to assess internal consistency of the OPAS (n= 102). The ocular pain intensity (24h and 2wks) and interference with quality of life (QoL) dimensions had good to excellent internal consistency. A p-value of less than 0.05 was considered statistically significant.
Responsiveness and Longitudinal Validity
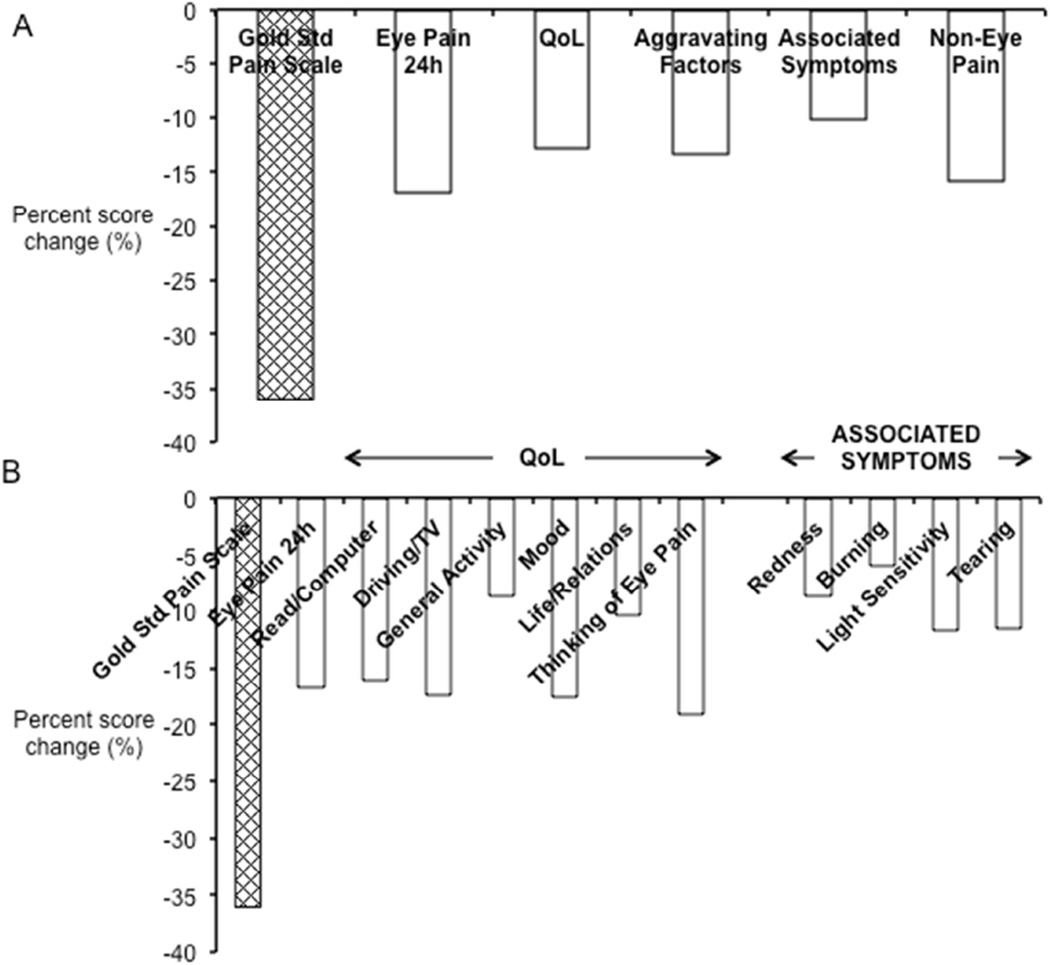
To assess responsiveness of the OPAS, pre- and post-treatment composite scores of 21 subjects were monitored and reviewed for the gold standard pain rating scale and each dimension of the OPAS. Follow-up composite scores reflected an improvement in every dimension of the OPAS following reduction in ocular pain intensity (Figure 3A): Wong-Baker FACES® Pain Rating Scale (initial visit: 3.83±2.63, follow-up visit: 2.45±2.80, Δ= −1.38±2.42, n= 21), 24h–ocular pain intensity (initial visit: 3.34±2.48, follow-up visit: 2.83±2.84, Δ= −0.57±2.01, n= 21), interference in quality of life (initial visit: 5.10±3.47, follow-up visit: 4.45±3.65, Δ= −0.65±2.06, n= 21), impact of aggravating factors (initial visit: 3.95±2.90, follow-up visit: 3.43±2.92, Δ= −0.52±2.67, n=21), accompanying associated symptoms (initial visit: 6.58±3.00, follow-up visit: 5.92±3.09, Δ= −0.67±2.42, n=19) and non-ocular pain (initial visit: 2.51±2.23, follow-up visit: 2.11±2.52, Δ= −0.39±2.73, n=21). In addition to composite scores for each dimension of the OPAS (Figure 3A), detailed deconstructed information was also available for constituents of each dimension (Figure 3B) displaying the pattern of responsiveness within the dimensions of quality of life and associated symptoms. With an average 36.0% reduction in ocular pain intensity as measured by the Wong-Baker FACES® Pain Rating Scale, the test scale 24-hour ocular pain intensity scores improved by 16.8% on average with concomitant 12.8% improvement on average in QoL most notably seen in the subjects’ preoccupation with eye pain (19.1%), mood (17.5%), ability to drive and watch television (17.4%), ease of reading and using the computer (16.10%) and relationships with people around them (10.4%). Furthermore, with reduction in ocular pain, subjects also reported less aggravation of symptoms by mechanical factors (22.1%) and fewer associated symptoms of light sensitivity (11.7%) and tearing (11.5%). There was direct correlation between the percent changes in 24-hour ocular pain intensity and quality of life scores (rp = 0.63, n= 17, P= 0.007). These results quantitatively elucidate the relationship of ocular surface pain with activities of daily life using the OPAS.
Figure 3. Responsiveness of the OPAS.
Dimension scores from the first follow-up visit post-treatment (n=21) were analyzed for determining responsiveness of the OPAS. Percent changes in mean scores showed that (A) there was reduction in eye pain intensity as measured by the gold standard (Gold Std) pain intensity scale (the Wong-Baker FACES® Pain Rating Scale) with improvement in all dimensions of the OPAS seen by concurrent reduction in mean dimension scores for eye pain intensity (past 24 hours), impact on quality of life (QoL), aggravating factors, associated symptoms and non-eye pain. (B) Detailed information was also retrieved by reviewing scores from individual test questions within each dimension. Within the dimensions of QoL and associated symptoms, sub-scale information regarding varying degrees of improvement in specific activities of daily life and eye-pain associated symptoms were also seen with reduction in eye pain intensity. Therefore, the OPAS in its entirety is responsive to changes across all dimensions.
Longitudinal validity of the 24-hour ocular pain intensity scale was confirmed by a highly significant correlation of mean change in scores between the 24-hour ocular pain intensity scale and the Wong-Baker FACES® Pain Rating Scale (rs= 0.69, P= 0.0006, n= 21).
Sensitivity, Specificity, Likelihood Ratios, Predictive Values, Accuracy
Given that ocular pain intensity was the primary outcome measure of the OPAS, it was necessary to establish the diagnostic utility of this tool. We determined diagnostic properties of the ocular pain intensity (24-hour) dimension of the OPAS and adjusted for the prevalence of ocular pain in our study population (79.4%). Table 6 summarizes these properties and demonstrates that the 24-hour ocular pain intensity scale of the OPAS is highly sensitive (93.8%), specific (80.9%), and accurate (91.2%) in detecting patients with ocular pain. Prevalence-independent diagnostic variables such as diagnostic odds ratio (DOR>50), and positive likelihood ratio (LR+ >10) supported the clinical usefulness of the OPAS in detecting ocular pain.30
Table 6.
Diagnostic properties of the Ocular Pain Assessment Survey (OPAS) for measuring ocular pain intensity.
| 95% Confide nce Interval | |||
|---|---|---|---|
| Lower limit | Upper limit | ||
| Prevalence | 0.79 | 0.70 | 0.87 |
| Sensitivity | 0.94 | 0.86 | 0.98 |
| Specificity | 0.81 | 0.57 | 0.94 |
| PPV | 0.95 | 0.87 | 0.98 |
| NPV | 0.77 | 0.54 | 0.91 |
| ^LR+ | 19 | 7.30 | 49.45 |
| ^LR- | 0.29 | 0.13 | 0.65 |
| DOR | 64.59 | ||
| Accuracy | 0.91 | ||
The prevalence of ocular pain in the study population was 79%. The OPAS was 94% sensitive, 81% specific and 91% accurate in measuring ocular pain intensity as compared to the gold standard pain rating scale. The OPAS had high positive and negative predictive values (PPV, NPV), high positive likelihood ratio (LR+ >10), a lower negative likelihood ratio (LR-) and a diagnostic odds ratio (DOR= LR+/LR-) greater than 50 suggesting that the OPAS was a valuable test for measuring ocular pain intensity.30
DISCUSSION
In this paper we present a quantitative ocular pain-specific questionnaire that is valid, reliable, responsive, multi-dimensional, and can accurately detect and assess ocular pain. The OPAS offers both composite and individual scores for each of its 6 dimensions that can be calculated at baseline and monitored over the course of treatment of ocular pain. This feature of the OPAS is particularly novel and of importance, as it not only allows monitoring ocular pain intensity, which may be a measure of disease severity, but also its impact on related dimensions such as quality of life, aggravating factors and associated symptoms. We tested the OPAS in patients that presented to the cornea clinic; the pain patients in our study had corneal and ocular surface pain resulting from keratitis, corneal ulcers, dry eye disease, conjunctivitis, keratoconus and refractive surgery. The 24-hour ocular pain intensity dimension was valid, reliable, internally consistent and responsive to treatment, which correlated with the response in the current gold standard pain intensity score. It also exhibited sensitivity, specificity, and accuracy in detecting ocular pain with high likelihood ratios and predictive values, further adding diagnostic utility to the OPAS. These features of the ocular pain intensity dimension of the OPAS make it a highly versatile tool for both clinical practice and clinical trials alike, where objective metrics of ocular pain assessment have been an unmet demand. However, it is important to note that predictive values of the OPAS in our study are specific to the prevalence of ocular pain at a given point in time; the prevalence of corneal and ocular surface pain is likely to vary between time points even within the same center.
An interesting and potentially insightful aspect of the OPAS is its non-ocular pain dimension. Pain and discomfort associated with dry eye disease is accompanied by affective disorders such as anxiety and major depression,31–33 which can manifest as non-ocular pain such as headaches and body aches. Furthermore, patients with dry eye disease also have high pain sensitivity and low pain tolerance to non-ocular stimuli.34 It is this rationale that led us to incorporate a domain on non-ocular pain, measuring both intensity and pre-occupation with pain. Recently, Galor and colleagues showed that non-ocular pain not only correlated with dry eye symptoms but also attributed to its variability,35 making it an important component to measure and investigate in future dry eye studies. Here we demonstrate that the OPAS provides a reliable and responsive tool, with which we can quantify non-ocular pain intensity.
In the context of ocular pain and its impact on quality of life and non-ocular pain, we observed that ocular pain intensity significantly correlated with both an impact on quality of life, and non-ocular pain. Moreover, with improvement in acute ocular pain intensity there was concomitant improvement in both QoL, and non-ocular pain. One of the benefits of the OPAS is that each dimension can be further deconstructed into its constituent scales to generate more in-depth trends if deemed necessary. For example, within the QoL dimension, we noted that some of its sub-scales showed greater improvement. In particular, improvement in scores was seen in the time spent thinking about eye pain, mood, the ability to drive and watch TV, as well as reading or using the computer. We therefore also quantified the relationship between corneal pain and quality of life using a validated ocular-pain specific tool. Thus, the OPAS may have relevance to clinical trials that seek meaningful endpoints both in terms of pain intensity and its translational impact on activities of daily life.
Of particular interest to corneal pain neurobiology will be the dimensions on aggravating factors and associated symptoms. Sensory nerves of the cornea are classified based on the stimuli that activate their nerve endings; 70% of corneal sensory nerves have polymodal receptors and as the name suggests, these nerves respond to mechanical, heat and chemical stimuli; cold receptors are found on 10–15% of corneal sensory nerves that respond to tear film evaporation and cold air; mechano-nociceptors are present on 20% of all sensory nerve endings and they fire in response to mechanical contact.36–38 Therefore, by determining which sub-scales of the aggravating factors dimension are affected the most (mechanical vs. chemical), it may lend some clue to the type of nociceptors and signaling pathways involved. Most ocular pain patients complain of light sensitivity or photoallodynia.39 It has been shown that light-induced trigeminal sensitization can induce photoallodynia.40 Therefore it is plausible that the previously sensitized corneal sensory nerve endings of corneal pain patients are more vulnerable to light-induced sensitization leading to photoallodynia associated with corneal pain or corneal neuropathy. Consequently, as the pain subsides and the nerve endings de-sensitize, they become less vulnerable to light-sensitization as well, experienced as reduced photoallodynia. In keeping with this theory, we observed that with improvement in ocular pain intensity, there was an accompanying reduction in sensitivity to light. Sensitivity to light saw the most pronounced change among the associated symptoms in response to improved corneal pain.
When assessing patients with corneal pain, it becomes necessary to differentiate physiological nociceptive ocular pain from neuropathic ocular pain, as the trajectory of management and nature of treatment (local vs. systemic) varies greatly between the two subsets of ocular surface pain.41–44 Pain is a physiologic, protective response of the nervous system to a noxious stimulus. However, in some cases due to maladaptive pain responses and neuroplasticity, there is peripheral or central sensitization of the nociceptive pathways leading to neuropathic pain, which is chronic, unexplained by signs or history, and accompanied by one or more of the following features: hyperalgesia, allodynia, spontaneous pain, dysesthesia, burning, and irritation.45–48 Given the importance of distinguishing neuropathic pain from physiological nociceptive pain, numerous questionnaires have been developed and validated to screen for non-ocular neuropathic pain: Neuropathic Pain Symptom Inventory, NPSI;49–52 Leeds Assessment of Neuropathic Symptoms and Signs, LANSS;53–56 Neuropathic Pain Questionnaire, NPQ; DN4 Pain;57–61 painDETECT;62–67 and ID Pain.68–70 Subtypes of neuropathic pain can also be detected using the modified Neuropathic Pain Symptom Inventory in German, NPSI-G.71 Recently, seve ghlighted the role of neuropathic pain in patients with chronic dry eye-like pain.42,43,72 Therefore, there is considerable interest and benefit to developing and validating a questionnaire specifically for screening neuropathic ocular pain. The OPAS may be used as a tool to quantify pain intensity, measure the impact of pain on affect and activities of daily life (quality of life), and provide a quantitative metric to track symptom relief among patients with neuropathic ocular pain.
This study has some limitations: first, the study pain population only comprised of patients with corneal pain, making it a necessary next step to test and validate the OPAS in other conditions with other causes of ocular pain such as glaucoma and uveitis; second, diagnostic properties such as the predictive values are dependent on prevalence of disease and will most likely differ between populations based on the prevalence of ocular pain; third, we performed exploratory factor analysis since our goal was to identify the structure of the OPAS without making assumptions a priori. Therefore, future studies will need to perform confirmatory factor analysis to establish whether or not the OPAS remains multi-dimensional when tested in other disease groups as well.
In conclusion, the availability of a validated and ocular pain-specific quantitative questionnaire that also allows objective measurement of pain and quality of life may spur clinical trials in ocular pain drug development. Clinicians may now be better equipped to quantify and monitor ocular pain with the aid of pain scores to guide patient-centered treatment strategies.
Supplementary Material
Acknowledgments
We thank Dr. Perry Rosenthal (Boston Eye Pain Foundation) and Dr. Joseph Ciolino (Cornea Service, Department of Ophthalmology, Massachusetts Eye and Ear Infirmary) for valuable discussions on this topic. We would also like to thank the organizations for funding our research: NIH K08-EY020575 (PH) and MEEI Foundation grant (PH), Research to Prevent Blindness Career Development Award (PH), Falk Medical Research Trust (PH). The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentation: This work has been presented in part at the 28th biennial cornea conference in Boston, MA on October 17, 2013 and at the annual ARVO meeting in Orlando, FL on May 4, 2014.
Financial interest: No conflicting relationship exists for any author.
REFERENCES
- 1.Roberts CJ, MacLeod JD, Elkington AR. Ocular pain: a casualty study. The spectrum and prevalence of pain in acute eye disease. Eye. 1997;11(Pt 3):342–344. doi: 10.1038/eye.1997.72. [DOI] [PubMed] [Google Scholar]
- 2.Rosenblatt MA, Sakol PJ. Ocular and periocular pain. Otolaryngologic clinics of North America. 1989;22:1173–1203. [PubMed] [Google Scholar]
- 3.Hitchings RA. The symptom of ocular pain. Transactions of the ophthalmological societies of the United Kingdom. 1980;100:257–259. [PubMed] [Google Scholar]
- 4.Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 5.NEI. Summary of white papers by NEI Workshop on Ocular Pain and Sensitivity Participants, Strategic Planning (NEI) Department of Health and Human Services, National Institutes of Health; 2015. [Google Scholar]
- 6.Radnovich R, Chapman CR, Gudin JA, et al. Acute pain: effective management requires comprehensive assessment. Postgrad Med. 2014;126:59–72. doi: 10.3810/pgm.2014.07.2784. [DOI] [PubMed] [Google Scholar]
- 7.Gulati A, Sullivan R, Buring JE, et al. Validation and repeatability of a short questionnaire for dry eye syndrome. Am J Ophthalmol. 2006;142:125–131. doi: 10.1016/j.ajo.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 8.Gothwal VK, Pesudovs K, Wright TA, McMonnies CW. McMonnies questionnaire: enhancing screening for dry eye syndromes with Rasch analysis. Invest Ophthalmol Vis Sci. 2010;51:1401–1407. doi: 10.1167/iovs.09-4180. [DOI] [PubMed] [Google Scholar]
- 9.Nichols KK, Nichols JJ, Mitchell GL. The reliability and validity of McMonnies Dry Eye Index. Cornea. 2004;23:365–371. doi: 10.1097/00003226-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 11.Ngo W, Situ P, Keir N, et al. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea. 2013;32:1204–1210. doi: 10.1097/ICO.0b013e318294b0c0. [DOI] [PubMed] [Google Scholar]
- 12.Amparo F, Schaumberg DA, Dana R. Comparison of Two Questionnaires for Dry Eye Symptom Assessment: The Ocular Surface Disease Index and the Symptom Assessment in Dry Eye. Ophthalmology. 2015;122:1498–1503. doi: 10.1016/j.ophtha.2015.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaumberg DA, Gulati A, Mathers WD, et al. Development and validation of a short global dry eye symptom index. Ocul Surf. 2007;5:50–57. doi: 10.1016/s1542-0124(12)70053-8. [DOI] [PubMed] [Google Scholar]
- 14.Mangione CM, Lee PP, Pitts J, et al. Psychometric properties of the National Eye Institute Visual function Questionnaire (NEI-VFQ). NEI-VFQ Field Test Investigators. Arch Ophthalmol. 1998;116:1496–1504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- 15.Nichols KK, Mitchell GL, Zadnik K. Performance and repeatability of the NEI-VFQ-25 in patients with dry eye. Cornea. 2002;21:578–583. doi: 10.1097/00003226-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Chambers CT, Giesbrecht K, Craig KD, et al. A comparison of faces scales for the measurement of pediatric pain: children's and parents' ratings. Pain. 1999;83:25–35. doi: 10.1016/s0304-3959(99)00086-x. [DOI] [PubMed] [Google Scholar]
- 17.Chibnall JT, Tait RC. Pain assessment in cognitively impaired and unimpaired older adults: a comparison of four scales. Pain. 2001;92:173–186. doi: 10.1016/s0304-3959(00)00485-1. [DOI] [PubMed] [Google Scholar]
- 18.Jensen MP, Karoly P, O'Riordan EF, et al. The subjective experience of acute pain. An assessment of the utility of 10 indices. Clin J Pain. 1989;5:153–159. doi: 10.1097/00002508-198906000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Closs SJ, Barr B, Briggs M, et al. A comparison of five pain assessment scales for nursing home residents with varying degrees of cognitive impairment. J Pain Symptom Manage. 2004;27:196–205. doi: 10.1016/j.jpainsymman.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Foundation W-BF. Wong-Baker FACES Foundation; v. 2015. [Google Scholar]
- 21.Lukes AS, Roy KH, Presthus JB, et al. Randomized comparative trial of cervical block protocols for pain management during hysteroscopic removal of polyps and myomas. Int J Womens Health. 2015;7:833–839. doi: 10.2147/IJWH.S50101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas P, Falkner-Radler C, Wimpissinger B, et al. Needle size in intravitreal injections -pain evaluation of a randomized clinical trial. Acta Ophthalmol. 2015 doi: 10.1111/aos.12901. [DOI] [PubMed] [Google Scholar]
- 23.Frigerio A, Heaton JT, Cavallari P, et al. Electrical Stimulation of Eye Blink in Individuals with Acute Facial Palsy: Progress toward a Bionic Blink. Plast Reconstr Surg. 2015;136:515e–523e. doi: 10.1097/PRS.0000000000001639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang L, Zhang K, He W, et al. Perceived Pain during Cataract Surgery with Topical Anesthesia: A Comparison between First-Eye and Second-Eye Surgery. J Ophthalmol. 2015;2015:383456. doi: 10.1155/2015/383456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghanem VC, Ghanem RC, de Oliveira R. Postoperative pain after corneal collagen cross-linking. Cornea. 2013;32:20–24. doi: 10.1097/ICO.0b013e31824d6fe3. [DOI] [PubMed] [Google Scholar]
- 26.Yau GL, Jackman CS, Hooper PL, Sheidow TG. Intravitreal injection anesthesia--comparison of different topical agents: a prospective randomized controlled trial. Am J Ophthalmol. 2011;151:333–337. doi: 10.1016/j.ajo.2010.08.031. e2. [DOI] [PubMed] [Google Scholar]
- 27.Caudle LE, Williams KA, Pesudovs K. The Eye Sensation Scale: an ophthalmic pain severity measure. Optom Vis Sci. 2007;84:752–762. doi: 10.1097/OPX.0b013e31812f7690. [DOI] [PubMed] [Google Scholar]
- 28.Gawlicki MC, Reilly MC, Popielnicki A, Reilly K. Linguistic validation of the US Spanish work productivity and activity impairment questionnaire, general health version. Value Health. 2006;9:199–204. doi: 10.1111/j.1524-4733.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- 29.Foundation W-BF. FACES History. 1983;2016 [Google Scholar]
- 30.Jekel JF, Katz DL, Elmore JG. Epidemiology, Biostatistics, and Preventive Medicine. Philadelphia, PA: W.B. Saunders; 2001. p. 417. [Google Scholar]
- 31.van der Vaart R, Weaver MA, Lefebvre C, Davis RM. The Association Between Dry Eye Disease and Depression and Anxiety in a Large Population-Based Study. Am J Ophthalmol. 2015;159:470–474. doi: 10.1016/j.ajo.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim KW, Han SB, Han ER, et al. Association between depression and dry eye disease in an elderly population. Invest Ophthalmol Vis Sci. 2011;52:7954–7958. doi: 10.1167/iovs.11-8050. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Gong L, Sun X, Chapin WJ. Anxiety and depression in patients with dry eye syndrome. Curr Eye Res. 2011;36:1–7. doi: 10.3109/02713683.2010.519850. [DOI] [PubMed] [Google Scholar]
- 34.Vehof J, Kozareva D, Hysi PG, et al. Relationship between dry eye symptoms and pain sensitivity. JAMA Ophthalmol. 2013;131:1304–1308. doi: 10.1001/jamaophthalmol.2013.4399. [DOI] [PubMed] [Google Scholar]
- 35.Galor A, Felix ER, Feuer W, et al. Dry eye symptoms align more closely to non-ocular conditions than to tear film parameters. Br J Ophthalmol. 2015 doi: 10.1136/bjophthalmol-2014-306481. [DOI] [PubMed] [Google Scholar]
- 36.Acosta MC, Belmonte C, Gallar J. Sensory experiences in humans and single-unit activity in cats evoked by polymodal stimulation of the cornea. J Physiol. 2001;534:511–525. doi: 10.1111/j.1469-7793.2001.t01-1-00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acosta MC, Tan ME, Belmonte C, Gallar J. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol Vis Sci. 2001;42:2063–2067. [PubMed] [Google Scholar]
- 38.Carr RW, Pianova S, Fernandez J, et al. Effects of heating and cooling on nerve terminal impulses recorded from cold-sensitive receptors in the guinea-pig cornea. J Gen Physiol. 2003;121:427–439. doi: 10.1085/jgp.200308814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aggarwal S, Kheirkhah A, Cavalcanti BM, et al. Autologous Serum Tears for Treatment of Photoallodynia in Patients with Corneal Neuropathy: Efficacy and Evaluation with In Vivo Confocal Microscopy. Ocul Surf. 2015;13:250–262. doi: 10.1016/j.jtos.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dolgonos S, Ayyala H, Evinger C. Light-induced trigeminal sensitization without central visual pathways: another mechanism for photophobia. Invest Ophthalmol Vis Sci. 2011;52:7852–7858. doi: 10.1167/iovs.11-7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shetty R, Deshpande K, Ghosh A, Sethu S. Management of Ocular Neuropathic Pain With Vitamin B12 Supplements: A Case Report. Cornea. 2015;34:1324–1325. doi: 10.1097/ICO.0000000000000572. [DOI] [PubMed] [Google Scholar]
- 42.Galor A, Levitt RC, Felix ER, et al. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye (Lond) 2015;29:301–312. doi: 10.1038/eye.2014.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galor A, Batawi H, Felix ER, et al. Incomplete response to artificial tears is associated with features of neuropathic ocular pain. Br J Ophthalmol. 2015 doi: 10.1136/bjophthalmol-2015-307094. [DOI] [PubMed] [Google Scholar]
- 44.Kulkantrakorn K, Lorsuwansiri C, Meesawatsom P. 0.025% capsaicin gel for the treatment of painful diabetic neuropathy: a randomized, double-blind, crossover, placebo-controlled trial. Pain Pract. 2013;13:497–503. doi: 10.1111/papr.12013. [DOI] [PubMed] [Google Scholar]
- 45.Pascoal-Faria P, Yalcin N, Fregni F. Neural markers of neuropathic pain associated with maladaptive plasticity in spinal cord injury. Pain Pract. 2015;15:371–377. doi: 10.1111/papr.12237. [DOI] [PubMed] [Google Scholar]
- 46.Tan AM, Samad OA, Fischer TZ, et al. Maladaptive dendritic spine remodeling contributes to diabetic neuropathic pain. J Neurosci. 2012;32:6795–6807. doi: 10.1523/JNEUROSCI.1017-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickinson BD, Head CA, Gitlow S, Osbahr AJ., 3rd Maldynia: pathophysiology and management of neuropathic and maladaptive pain--a report of the AMA Council on Science and Public Health. Pain Med. 2010;11:1635–1653. doi: 10.1111/j.1526-4637.2010.00986.x. [DOI] [PubMed] [Google Scholar]
- 48.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villoria J, Rodriguez M, Berro MJ, et al. Psychometric validation of the neuropathic pain symptom inventory for its use in Spanish. J Pain Symptom Manage. 2011;42:134–146. doi: 10.1016/j.jpainsymman.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 50.Padua L, Briani C, Jann S, et al. Validation of the Italian version of the Neuropathic Pain Symptom Inventory in peripheral nervous system diseases. Neurol Sci. 2009;30:99–106. doi: 10.1007/s10072-009-0025-y. [DOI] [PubMed] [Google Scholar]
- 51.Crawford B, Bouhassira D, Wong A, Dukes E. Conceptual adequacy of the neuropathic pain symptom inventory in six countries. Health Qual Life Outcomes. 2008;6:62. doi: 10.1186/1477-7525-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouhassira D, Attal N, Fermanian J, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108:248–257. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 53.Spanos K, Lachanas VA, Chan P, et al. Validation of the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) questionnaire and its correlation with visual analog pain scales in Greek population. J Diabetes Complications. 2015 doi: 10.1016/j.jdiacomp.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Weingarten TN, Watson JC, Hooten WM, et al. Validation of the S-LANSS in the community setting. Pain. 2007;132:189–194. doi: 10.1016/j.pain.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 55.Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain. 2005;6:149–158. doi: 10.1016/j.jpain.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 56.Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92:147–157. doi: 10.1016/s0304-3959(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 57.Sykioti P, Zis P, Vadalouca A, et al. Validation of the Greek Version of the DN4 Diagnostic Questionnaire for Neuropathic Pain. Pain Pract. 2015;15:627–632. doi: 10.1111/papr.12221. [DOI] [PubMed] [Google Scholar]
- 58.Abdallah FW, Morgan PJ, Cil T, et al. Comparing the DN4 tool with the IASP grading system for chronic neuropathic pain screening after breast tumor resection with and without paravertebral blocks: a prospective 6-month validation study. Pain. 2015;156:740–749. doi: 10.1097/j.pain.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 59.van Seventer R, Vos C, Giezeman M, et al. Validation of the Dutch version of the DN4 diagnostic questionnaire for neuropathic pain. Pain Pract. 2013;13:390–398. doi: 10.1111/papr.12006. [DOI] [PubMed] [Google Scholar]
- 60.Perez C, Galvez R, Huelbes S, et al. Validity and reliability of the Spanish version of the DN4 (Douleur Neuropathique 4 questions) questionnaire for differential diagnosis of pain syndromes associated to a neuropathic or somatic component. Health Qual Life Outcomes. 2007;5:66. doi: 10.1186/1477-7525-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) Pain. 2005;114:29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 62.Cappelleri JC, Koduru V, Bienen EJ, Sadosky A. A cross-sectional study examining the psychometric properties of the painDETECT measure in neuropathic pain. J Pain Res. 2015;8:159–167. doi: 10.2147/JPR.S80046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cappelleri JC, Bienen EJ, Koduru V, Sadosky A. Measurement properties of painDETECT by average pain severity. Clinicoecon Outcomes Res. 2014;6:497–504. doi: 10.2147/CEOR.S68997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Timmerman H, Wolff AP, Schreyer T, et al. Cross-cultural adaptation to the Dutch language of the PainDETECT-Questionnaire. Pain Pract. 2013;13:206–214. doi: 10.1111/j.1533-2500.2012.00577.x. [DOI] [PubMed] [Google Scholar]
- 65.Gauffin J, Hankama T, Kautiainen H, et al. Neuropathic pain and use of PainDETECT in patients with fibromyalgia: a cohort study. BMC Neurol. 2013;13:21. doi: 10.1186/1471-2377-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Andres J, Perez-Cajaraville J, Lopez-Alarcon MD, et al. Cultural adaptation and validation of the painDETECT scale into Spanish. Clin J Pain. 2012;28:243–253. doi: 10.1097/AJP.0b013e31822bb35b. [DOI] [PubMed] [Google Scholar]
- 67.Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–1920. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 68.Reyes-Gibby C, Morrow PK, Bennett MI, et al. Neuropathic pain in breast cancer survivors: using the ID pain as a screening tool. J Pain Symptom Manage. 2010;39:882–889. doi: 10.1016/j.jpainsymman.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Paolo C, Di Nunno A, Vanacore N, Bruti G. ID migraine questionnaire in temporomandibular disorders with craniofacial pain: a study by using a multidisciplinary approach. Neurol Sci. 2009;30:295–299. doi: 10.1007/s10072-009-0098-7. [DOI] [PubMed] [Google Scholar]
- 70.Portenoy R. Development and testing of a neuropathic pain screening questionnaire: ID Pain. Curr Med Res Opin. 2006;22:1555–1565. doi: 10.1185/030079906X115702. [DOI] [PubMed] [Google Scholar]
- 71.Sommer C, Richter H, Rogausch JP, et al. A modified score to identify and discriminate neuropathic pain: a study on the German version of the Neuropathic Pain Symptom Inventory (NPSI) BMC Neurol. 2011;11:104. doi: 10.1186/1471-2377-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chao C, Golebiowski B, Stapleton F. The role of corneal innervation in LASIK-induced neuropathic dry eye. Ocul Surf. 2014;12:32–45. doi: 10.1016/j.jtos.2013.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.