Summary
Metabolic flexibility is the ability to respond or adapt to conditional changes in metabolic demand. This broad concept has been propagated to explain insulin resistance and mechanisms governing fuel selection between glucose and fatty acids, highlighting the metabolic inflexibility of obesity and type 2 diabetes. In parallel, contemporary exercise physiology research has helped to identify potential mechanisms underlying altered fuel metabolism in obesity and diabetes. Advances in ‘omics’ technologies have further stimulated additional basic and clinical-translational research to further interrogate mechanisms for improved metabolic flexibility in skeletal muscle and adipose tissue with the goal to prevent and treat metabolic disease.
What is metabolic flexibility?
Metabolic flexibility describes the ability of an organism to respond or adapt according to changes in metabolic or energy demand as well as the prevailing conditions or activity. It was first used as a term describing the increased capacity of helminthes, a parasitic worm, to generate chemical energy and key metabolites either aerobically or by using anaerobic respirations to give it greater versatility and metabolic flexibility to respond and adapt to environmental changes in its habitat (Kohler, 1985).
The more common concept of metabolic flexibility has been promulgated in the context of fuel selection in the transition from fasting to fed states, or fasting to insulin stimulation to explain insulin resistance (Goodpaster and Kelley, 2008). The original Randle Cycle (Randle et al., 1963) was put forth as a tenet to explain elevated fatty acid oxidation and reduced glucose oxidation underlying insulin resistance and type 2 diabetes. Kelley and Mandarino later reconsidered these concepts following a series of elegant in vivo limb balance studies demonstrating the metabolic inflexibility in human type 2 diabetes and obesity in which, during post-absorptive conditions, skeletal muscle has elevated glucose oxidation and reciprocal reduced fatty acid oxidation (Kelley, 1994, 1993; Kelley and Mandarino, 1990; Kelley et al., 1993). Since those first experiments were described, the term metabolic flexibility has evolved to encompass other metabolic circumstances and tissues and more broadly refers to a physiological adaptability. Metabolic flexibility was also inferred to have tissue specificity in response to nocturnal and diurnal fasted and fed conditions (Kelley et al., 1999).
Exercise is another physiological condition requiring metabolic flexibility in order to match fuel availability with the metabolic machinery to meet enormous increases in energy demands. Exercise duration and intensity can each profoundly influence energy demand, thereby taxing energy stores and catabolic pathways in very different ways. Although the topic of exercise-induced changes in metabolism has been covered in recent reviews (see (Egan and Zierath, 2013; Hawley et al., 2014)), the mechanisms underlying metabolic flexibility with exercise deserves further inquiry. “Muscle plasticity” was first used (Pette, 1980) as a term used to characterize muscle’s ability to respond to a variety of stimuli, and included a metabolic flexibility. Exercise training can alter fuel storage and availability, and recent evidence that exercise promotes changes in the skeletal muscle epigenome (Rasmussen et al., 2014), transcriptome (Keller et al., 2011; Raue et al., 2012) and proteome (Hoffman et al., 2015), all of which constitute an anabolic flexibility in order to meet changes in energy requirements for each bout of exercise or activity, merit deeper investigations into the molecular mechanisms driving metabolic flexibility.
Any review or discussion of these general concepts of metabolic flexibility deserves to be placed in some context and framework; for without this, the review could be too broad and unwieldy. We will review the processes and some of the underlying mechanisms of healthy metabolically flexible responses to fasting and feeding and from rest to exercise, and with some inferences to metabolic inflexibility as it relates to pathobiology. Within this context, we will review the evidence that exercise training can improve metabolic flexibility, highly relevant to improving the pathophysiological aspects of obesity, type 2 diabetes and aging. We will also attempt to summarize the evidence comparing and contrasting the effects of exercise training and calorie restriction-induced weight loss on metabolic flexibility, and the implications that this likely has on prevention and treatment of these conditions.
We emphasize the role of skeletal muscle and adipose tissue in metabolic flexibility in humans. These are two tissues, which play a crucial role in energy metabolism, and both can be accessed in humans with biopsies to interrogate their biology and response to acute and chronic interventions. Regardless of tissue, metabolic flexibility is driven by cellular and organelle processes, perhaps most pertinently in the mitochondria. Here we deliberate metabolic flexibility during relevant conditions of fasting, insulin stimulation and exercise. We also discuss some of the cellular aspects of metabolic flexibility that have been re-capitulated in vitro.
Fasting to feeding - Insulin resistance as part of the metabolic inflexibility in obesity and type 2 diabetes
Skeletal muscle drives fuel catabolism
The original limb balance indirect calorimetry technique established by Andres and colleagues in 1956 measured glucose and fatty acid oxidation via the Respiratory Quotient (RQ) of the forearm muscle during post-absorptive conditions (Andres et al., 1956). They clearly demonstrated that that the normal, healthy transition from fasting to feeding involves shifts in fuel selection from predominantly oxidative fatty acid metabolism to more glucose oxidation in skeletal muscle. Kelley and colleagues later demonstrated that this shift also included, albeit to a quantitatively lesser degree, increases in glycolytic energy production (Kelley et al., 1999).
Since energy expenditure, mostly from the thermic effect of food, increases by less than 10% (Acheson et al., 1984), this substrate shift serves to efficiently utilize energy sources based on the content or mixture of the macronutrients in the meal. The primary purpose of this substrate shift is to move from catabolic to anabolic processes in which energy can be effectively stored in skeletal muscle, adipose and liver tissues. Insulin release in response to a meal is a major driver of this shift.
Much of the attention around metabolic flexibility is because of its implication in insulin resistance, a concept first advanced by Wilhelm Falta and published in Vienna in 1931 as a possible underlying cause of type 2 diabetes (Falta and Boller, 1931). During the ensuing 85 years insulin resistance has evolved to become generally accepted as the predominant factor leading to type 2 diabetes, and the most probable single link among a constellation of cardiometabolic risk factors known as the metabolic syndrome linking obesity, type 2 diabetes and cardiovascular disease (Reaven, 1988).
Skeletal muscle insulin resistance and fatty acid metabolism
Insulin resistance is a key component of the metabolic inflexibility that can develop in many tissues and organs. The cellular mechanisms for insulin resistance have been reviewed extensively (Flier et al., 1979; Holland and Summers, 2008; Shulman, 2004). A substantial emphasis on mechanisms underlying insulin resistance in liver and skeletal muscle has been placed on the roles of impaired mitochondrial fatty acid oxidation and excess accumulation of lipid metabolites diacylglycerol and ceramides.
Skeletal muscle accounts for ~60–80% of the increase in glucose metabolism in response to insulin (Ng et al., 2012), and an enormous body of work supports a causal role for skeletal muscle insulin resistance in type 2 diabetes (DeFronzo and Tripathy, 2009; Petersen et al., 2007). Intuitively, a reduction in the amount of glucose entering the muscle cells and adipocytes from the bloodstream, along with a reduced suppression of hepatic glucose production, will elevate glucose in the blood in the absence of a corresponding increase in insulin release from the pancreatic beta cells. Diabetes develops, as the argument goes, if and when the beta cells fail to appropriately compensate for this insulin resistance with higher insulin secretion.
It is difficult to argue against insulin resistance in muscle, adipose tissue and liver causing hyperglycemia with inappropriate or failed beta cell compensation. Insulin resistance often precedes hyperinsulinemia and hyperglycemia (DeFronzo and Tripathy, 2009). There is, however, some debate about whether insulin resistance in muscle is a primary defect or an adaptation in diabetes. Defects in fatty acid oxidation (Kelley et al., 1999; Koves et al., 2008; McGarry, 1992), altered mitochondrial energetics (Lee et al., 2010; Morino et al., 2005) and intramyocellular lipid accumulation (Amati et al., 2011; Coen et al., 2010) have all been associated with insulin resistance and type 2 diabetes. Contentious debate also continues concerning which tissue or organ is the primary instigator of whole body insulin resistance, glucose intolerance and diabetes. Turner et al. have provided elegant time course evidence in a high-fat-fed rodent model of insulin resistance whereby hepatic insulin resistance precedes both adipose tissue and skeletal muscle insulin resistance (Turner et al., 2013). This study also highlights the important role of dysregulated fatty acid metabolism and lipid excess causing insulin resistance in these insulin-sensitive tissues. Additional evidence in humans suggests that hepatic and skeletal muscle insulin resistance could occur concomitantly (Chen et al., 2015). It is therefore likely that the initial insult to cause whole body insulin resistance and diabetes could originate within different tissues.
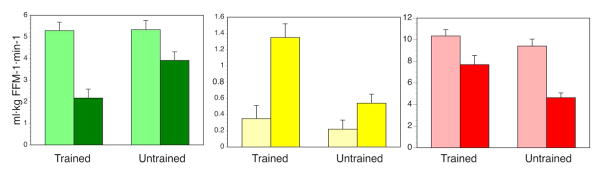
Regardless of whether or not insulin resistance in muscle can cause type 2 diabetes, it is likely that insulin resistance is part of an overall metabolic inflexibility that also encompasses defects in fatty acid metabolism. From the cellular perspective, excess glucose entering and stored in the muscle cell in the absence of increased energy expenditure could be harmful. Is insulin resistance then an adaptive response? There are certainly physiological conditions in which insulin resistance develops, which is not pathobiology. Prolonged fasting induces skeletal muscle insulin resistance parallel to elevated fatty acid oxidation (Hoeks et al., 2010). Similarly, lipid overload, often used as a model for insulin resistance (Brehm et al., 2006; Itani, 2002; Yu, 2002), may represent a model of metabolic flexibility rather than revealing a pathological mechanism of insulin resistance. In support of this, Phelix et al. and Dube et al. demonstrated in independent studies (Dube et al., 2014; Phielix et al., 2012) that endurance-trained athletes who have a high oxidative capacity in muscle can increase fatty acid oxidation in response to lipid overload, but they preserve glycogen storage within muscle at the expense of decreasing glucose oxidation (Figure 1, data obtained from (Dube et al., 2014)). This enhanced metabolic flexibility was associated with a higher mitochondrial capacity in exercise-trained muscle (Dube et al., 2014; Phielix et al., 2012). Recent evidence also suggests that circadian variation in the molecular metabolic machinery can influence metabolic flexibility (Bass and Lazar, 2016). This is an emerging area of investigation that will help to clarify the role of insulin resistance and metabolic flexibility in human health and disease.
Figure 1. Acute lipid oversupply during hyperinsulinemia reveals metabolic flexibility in trained compared to untrained subjects.
During a hyperinsulinemic-euglycemic “glucose” clamp without (light bars) or with (dark bars) co-infusion of intralipid, trained subjects decrease glucose oxidation (green bars), increase fatty acid oxidation (yellow bars) and preserve muscle glycogen storage (red bars) relative to untrained subjects, who exhibit metabolic inflexibility. In other words, untrained subjects do not effectively decrease glucose oxidation or increase fatty acid oxidation, and they have diminished glycogen storage in the face of lipid overload. Data were obtained from (Dube et al., 2014).
White adipose tissue orchestrates fuel flux
Compared to skeletal muscle, relatively little research is reported on metabolic flexibility of white adipose tissue (WAT). WAT has been historically regarded as a lipid reservoir; however, WAT is becoming increasingly recognized for playing an active role in lipid and glucose metabolism, as well as having the potential to increase thermogenesis (or ‘browning’) (Bostrom et al., 2012; Nedergaard and Cannon, 2014; Stanford et al., 2015). For purposes of this review, we focus on the inherent aspects of WAT metabolism and do not address the highly debated intricacies of WAT ‘browning’. WAT buffers circulating free fatty acids (FFAs) for peripheral tissues such as skeletal muscle and liver through a fine-tuned system of uptake, esterification and release of FFAs (so called triacylglycerol [TAG] cycling) (Reshef et al., 2003). This process requires – among many others – glycerol kinase, an enzyme that was thought to be absent in adipocytes prior to a ground-breaking in vitro study in 2002 (Guan et al., 2002). It is worth noting that TAG cycling also occurs within brown adipose tissue (BAT) (Yu et al., 2002), but metabolic flexibility of BAT relates more to TAG cycling linked with combustion within the cell rather than with storage and supply for peripheral tissues as is the case for WAT. While absence (Reitman and Gavrilova, 2000) or excess (obesity) of WAT are both associated with metabolic complications, a normal weight healthy woman can have as much WAT as an obese man with type 2 diabetes (Jensen, 2002). The WAT mass per se is therefore not the only culprit in obesity-driven metabolic abnormalities, highlighting the importance of healthy and metabolically adaptable WAT. Likewise, it is pertinent to note that visceral and subcutaneous adipose depots are highly correlated in cross-sectional studies (Fox et al., 2007), which makes it difficult to disentangle their individual contributions to metabolic health. The primary focus of this review is on abdominal subcutaneous adipose tissue, largely due to pragmatic issues around tissue availability in humans, but we will address differences between these two depots where appropriate.
Substantial evidence implicates elevated FFA levels [from inappropriate lipolysis] as a significant etiological factor for insulin resistance and type 2 diabetes (Eckel et al., 2005; Frayn and Coppack, 1992; Frayn et al., 1996; Randle et al., 1963; Savage et al., 2007). More contemporary studies, however, have refuted the relationship between elevated circulating FFAs and obesity-driven insulin resistance. To summarize these findings, a recent systematic analysis by Frayn and colleagues compared over 2,000 overweight/obese individuals with non-obese controls and found that the average difference between those two cohorts was modest (in the range of 70 μmol/L) and unrelated to fat mass (Karpe et al., 2011). In humans, the only significant site of FFA liberation is abdominal subcutaneous (i.e., upper-body) WAT with only a small and rather insignificant proportion arising from visceral adipose tissue (Nielsen et al., 2004). Transitioning from fasting to feeding elicits an insulin-stimulated suppression of lipolysis in WAT, a process that occurs via Akt-dependent and Akt-independent signaling pathways (Choi et al., 2010). In non-obese individuals, the EC50 of insulin to suppress lipolysis is half that required to suppress (hepatic) glucose output from the liver (Nurjhan et al., 1986). In the contrasting diabetic state, the amount of insulin required to completely suppress hepatic glucose production only achieves an 85% suppression of adipose lipolysis (Groop et al., 1989). These classical studies emphasize the sensitivity (and flexibility) of WAT to insulin in a healthy state and highlight its susceptibility to blunted insulin responsiveness as a potential early point of aberration in the etiology of whole body insulin resistance and type 2 diabetes.
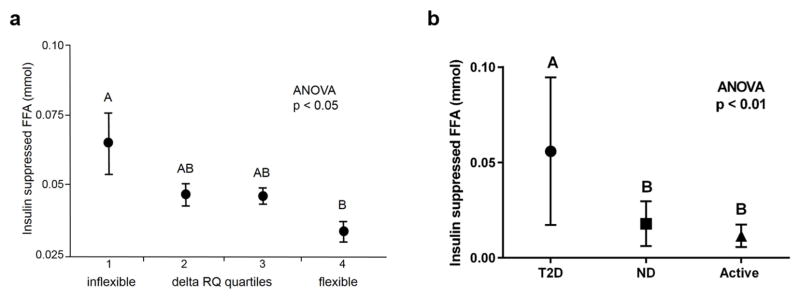
Figure 2 captures the power of WAT metabolic adaptability to influence metabolism in other tissues such as skeletal muscle. A blunted suppression of lipolysis by insulin during a hyperinsulinemic-euglycemic clamp is associated with reduced glycolytic catabolism and metabolic flexibility in healthy people (2a) (Sparks et al., 2009), and segregates by diabetes status, rather than fat mass (2b) (unpublished data). Overnight fasting elicits high lipolytic activity in WAT for a robust supply of FFAs (Frayn et al., 1996) and commensurately high rates fat oxidation in skeletal muscle (low RQ), an ability that is blunted in individuals with a family history of type 2 diabetes (Ukropcova et al., 2007). In the context of the insulin sensitivity of WAT (EC50) (Nurjhan et al., 1986), low levels of insulin are necessary for WAT to meet this challenge of fasting-induced demand for FFAs. When oscillations of insulin secretion are attenuated and/or absent, such as in people with a family history of type 2 diabetes (Matthews, 1996; O’Rahilly et al., 1988), WAT may develop insulin resistance as an adaptive response or defense mechanism in order to continue supply of FFAs to skeletal muscle and other tissues as needed. Pathobiology is therefore difficult to determine without conditional context.
Figure 2. Adipose tissue insulin responsiveness is critical for metabolic flexibility of other organs and is blunted in diabetes.
(a) Free fatty acids (FFAs) during a during a hyperinsulinemic-euglycemic clamp with a primed-continuous insulin infusion of 80 mU/m2/min for 3–4 hours are related to reduced metabolic flexibility (delta RQ) in a population of 56 healthy young men subdivided into quartiles of metabolic flexibility. (b) People with type 2 diabetes (T2D; n=18) have significantly higher levels of FFAs during an hyperinsulinemic-euglycemic clamp with a primed-continuous insulin infusion of 100 mU/m2/min for 3–4 hours compared with age- and BMI-matched healthy people (ND; n=6) and highly active people (Active; n=8). ANOVA was used to test for differences across quartiles of metabolic flexibility (delta RQ), with post hoc testing by mean equality contrast between different groups using the Tukey–Kramer HSD; alpha = 0.05. Type I error rate was set a priori at p < 0.05. Data are shown as means ± SEM. Levels which do not share the same letter are significantly different. FFAs were measured by high-performance liquid chromatography (HPLC) for (a) and by enzyme assay for (b).
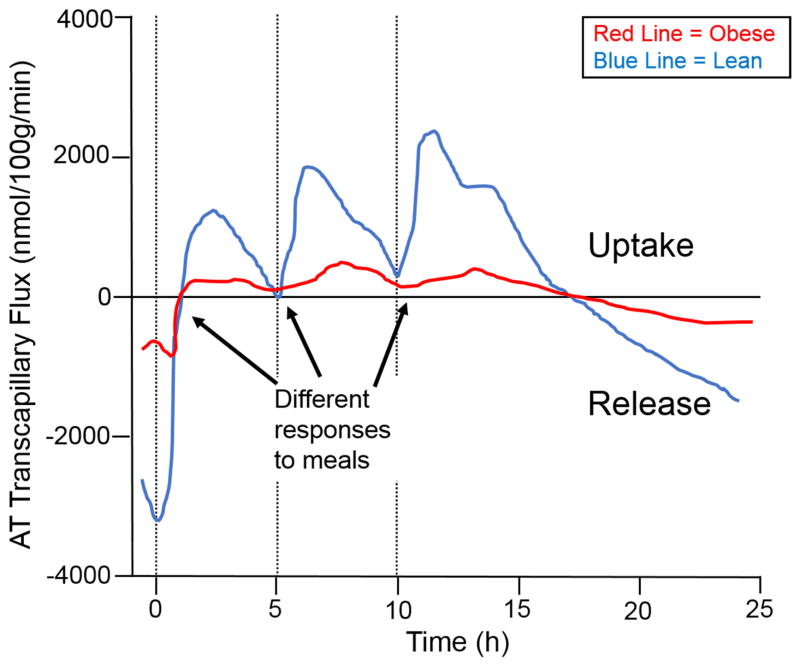
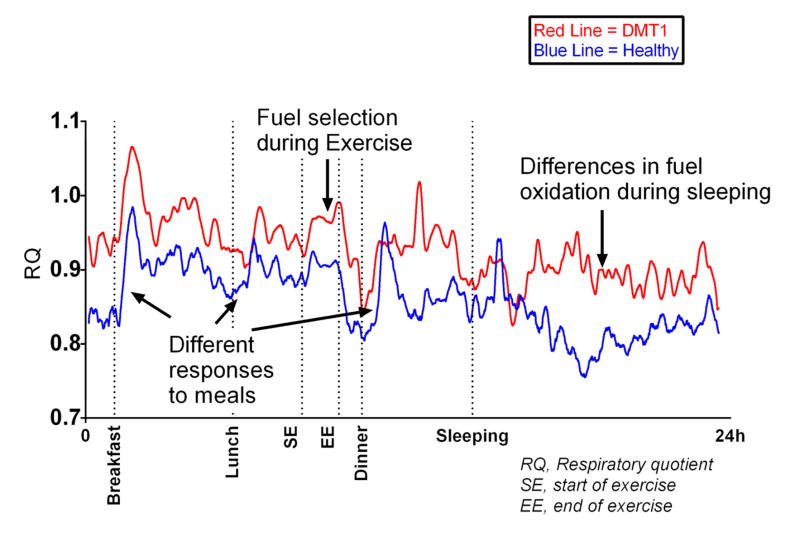
Considerable re-esterification of FFAs in WAT occurs during periods of active lipolysis such as fasting; in humans fasted for 60h, ~40% of liberated FFAs is recycled back into TAG in the subcutaneous WAT depot (Jensen et al., 2001), and the rest of the FFAs are released from WAT into circulation for catabolism by other tissues, typically skeletal muscle. WAT possesses a remarkable adaptability in obesity to expand and continuously store FFAs inertly as its innate function, which can often be perturbed in obesity-driven insulin resistance and results in down-regulation of basal (fasting) (Campbell et al., 1994; Horowitz et al., 1999) and dietary fat storage (McQuaid et al., 2011). Thiazolidinediones (TZDs) markedly improve insulin sensitivity and glucose homeostasis by expanding WAT (Girard, 2001) via a peroxisome proliferator-activated receptor (PPAR)-γ-induced rise in GyK levels and TAG cycling under conditions of fasting and feeding and eliminate reliance on glucose for such processes (Guan et al., 2002; Lehmann et al., 1995). Re-esterification is most prominent upon ingestion of a mixed meal when insulin induces the switch from FFA release to storage. Figure 3 elegantly illustrates the anabolic capacity of WAT. Over the course of three meals in a 24hr period, abdominally obese men have significantly lower transcapillary flux of FA (net fat storage and release) from WAT (McQuaid et al., 2011). Intuitively, as less dietary FFAs are progressively stored in WAT with each meal, these FFAs remain in circulation and are likely deposited ectopically in other tissues and lead to metabolic perturbations therein. Figure 4 depicts a progressive increase in post-meal RQ (so burning more carbohydrate than fat) by the 3rd of 3 meals over a 24hr period in lean healthy individuals (unpublished data). The essence of coordinated metabolic flexibility among tissues in the healthy state dictates that the more fat that is stored (and inertly sequestered) in WAT post-meal, the less fat that is available for catabolism by other tissues leading to more reliance on carbohydrate oxidation (higher RQ).
Figure 3. Transcapillary flux of FFAs is reduced with obesity.
The release of free fatty acids (FFAs) and the extraction of triglycerides from plasma (nmol/100g/min) in abdominal subcutaneous white adipose tissue (WAT) is significantly lower in the WAT of abdominally obese men (red line) compared with lean men (blue line) (both time x group, p < 0.001) across consumption of three mixed meals over the course of 24 hours. Meal consumption is indicated by a dashed line.
Figure 4. Respiratory quotient (RQ) kinetic during 24 hours in a metabolic chamber.
Lines represent individual responses for a type I diabetes mellitus patient (DMT1, red line) and a healthy volunteer (blue line). Arrows indicate different features of metabolic flexibility (exercise, response to a meal and sleeping) as assessed in whole room respiratory chamber.
Rest to exercise – Fuel selection to support increased energy demand
Physical activity can dramatically increase energy expenditure and demand. Rigorous exercise can increase energy expenditure 25-fold compared to resting metabolic rate. The physiology and biochemistry of fuel selection during exercise has been the topic of investigation for several decades. The vast majority of human studies have been conducted in normal weight young subjects who typically have considerable metabolic flexibility in fuel selection. These concepts and efforts put forth to better understand fuel metabolism during exercise have also in large part been born out of the interest to improve sports performance. The literature is replete with studies aimed at strategies to prolong endurance by maintenance of higher rates of fatty acid oxidation (Jeukendrup et al., 1996; Jeukendrup et al., 1998a; Jeukendrup et al., 1998b; van Loon et al., 1999) and exogenous carbohydrate oxidation (Goodpaster et al., 1996; Horowitz et al., 1999) to preserve muscle glycogen stores, which has been demonstrated to limit performance.
Skeletal muscle accounts for more than 95% of energy requirements during moderate to vigorous exercise. Intramuscular glycogen, triglycerides, plasma glucose and plasma fatty acids (primarily from abdominal subcutaneous WAT lipolysis) combine to provide the necessary fuel to working muscle (Romijn, 1993). Thus exercise requires tremendous metabolic flexibility to increase energy supply from all of these sources to support the enormous energy demands of exercise primarily by skeletal muscle.
Many of the myocellular changes that occur during exercise, not surprisingly, are related to catabolism. Exercise is a powerful activator of AMPK (Jorgensen et al., 2006), which has been consistently reported to be a master energy sensor. Pharmacological activation of AMPK alters the expression of many of the same genes seen with exercise (Narkar et al., 2008). The sirtuin pathways have also been implicated in mechanisms of energy sensing in skeletal muscle and many other tissues requiring metabolic flexibility (Jing et al., 2011). Exercise acutely alters the molecular and biochemical machinery required to mobilize energy for carbohydrate and fatty acid oxidation. These changes in skeletal muscle promote greater energy supply. The metabolic flexibility to switch between glucose and fatty acid catabolism during acute exercise in healthy people is driven largely by the intensity and duration of exercise. Higher intensity exercise increasingly relies on glucose oxidation, through oxidative phosphorylation but more exclusively on anaerobic glycolysis during higher intensity exercise. This occurs independently of insulin (Goodyear and Kahn, 1998), as circulating insulin levels are normally very low during exercise. Fatty acid oxidation contributes quantitatively and proportionally less as exercise intensity increases (Brooks, 1997; Romijn, 1993). As exercise duration becomes longer, however, fatty acids make a greater contribution to overall energy supply (Jeukendrup, 2002).
One aspect of WAT metabolic flexibility is the ability to mobilize FFAs (primarily from abdominal subcutaneous WAT) in response to an acute exercise-mediated rise in catecholamines (Arner et al., 1990b). Visceral adipose tissue appears to be more responsive to adrenergic activation (Arner, 1995; Mauriege et al., 1987). Indeed, some studies have shown that the visceral (vs. subcutaneous) adipose depot has a greater relative change in response to exercise interventions (Schwartz et al., 1991; Thomas et al., 2000). The abdominal subcutaneous adipose depot is still considered the largest provider of plasma FFAs during an acute exercise bout with visceral providing a very small fraction since the visceral depot is much smaller in size (especially in the healthy state) (Horowitz, 2003). Even low-intensity exercise increases epinephrine concentration to about three-fold above basal (Henderson et al., 2007; McMurray et al., 1987). As the duration of fixed-intensity exercise increases, there is a rise in regional WAT lipolysis (Stallknecht et al., 2007; Stallknecht et al., 2001; Stich et al., 2000), which has been attributed to slow-acting hormones such as growth hormone (Divertie et al., 1991; Hansen et al., 2005; Healy et al., 2006). Selective blocking of β-adrenergic receptors in abdominal subcutaneous WAT during 30 minutes of moderate intensity exercise in lean individuals drastically reduces lipolysis during exercise (Arner et al., 1990b). To further illustrate this point, deletion of the key lipolytic enzyme adipose triglyceride lipase (ATGL) from adipocytes impairs acute exercise performance in mice due to reduced FFA supply to skeletal muscle (Dube et al., 2015), highlighting the critical need for WAT metabolic flexibility relative to other tissues. Deletion of ATGL from myotubes has no effect on exercise performance (Dube et al., 2015). Given the reduction of β2-adrenergic receptor density in adipocytes isolated from obese individuals (Arner et al., 1990a; Horowitz et al., 1999), it stands to reason that resistance to catecholamine action in WAT is one possible explanation for blunted fasting- and exercise-induced FFA release from WAT. The ability of WAT to liberate FFAs during acute bouts of exercise and over the course of repeated bouts of exercise (chronic training) plays an important role in supporting whole body fat oxidation, particularly in skeletal muscle.
In addition to increasing availability of fatty acids and glucose to energy production during activity, skeletal muscle also responds to acute exercise to prepare the organism for the next bout of activity. This, in many ways, precipitates an exercise-training response. These changes also pertain to catabolic processes, including autophagy (Mansueto et al., 2017) and other processes to promote organelle and cellular remodeling to enhance overall energy metabolism. Acute exercise induces epigenomic, transcriptomic and proteomic changes in skeletal muscle and WAT, which integrate changes in metabolic pathways to confer greater energy production and better metabolic flexibility for subsequent exercise bouts. Although the literature is extremely limited in this regard, chronic exercise augments methylation of CpG sites in genes related to lipogenesis and enriches expression of genes related with oxidative phosphorylation and protein synthesis in subcutaneous WAT of the leg (Ronn et al., 2013; Ronn et al., 2014). These recent studies highlight how the emergence and evolution of ‘omics’ technologies, and newer analytic methods, strategies and bioinformatics can be implemented to interrogate cellular changes in response to exercise.
Are some pathological conditions characterized by metabolic inflexibility during exercise?
Fuel selection during exercise in pathophysiological states such as obesity, insulin resistance and type 2 diabetes has received notably less attention. Despite having reduced fatty acid oxidation during resting, fasted conditions, subjects with obesity (Goodpaster et al., 2002; Horowitz and Klein, 2000) and type 2 diabetes (Colberg et al., 1996) have similar – or even elevated -- fatty acid oxidation during exercise. Moreover, patients with type 2 diabetes oxidize more plasma glucose during acute exercise (Colberg et al., 1996), which could help to explain part of the glucose lowering effects of exercise and activity in diabetes. Figure 4 highlights the ability to precisely and accurately quantify inter-individual differences as well as acute changes in human whole body fuel selection employing 24-hour whole-room calorimetry (unpublished data).
Few studies have investigated whether myocellular responses or changes during acute exercise vary according to aging, obesity or diabetes. Mandarino et al. reported that exercise-induced changes in gene expression are dependent upon skeletal muscle insulin sensitivity of the subject (McLean et al., 2015). In vitro studies could provide more mechanistic insight into acute exercise responses, since many of the metabolic phenotypes observed in vivo are preserved in culture in human satellite cells isolated from skeletal muscle (reviewed in (Aas et al., 2013)). For example, Ukropcova et al. demonstrated an intrinsic metabolic flexibility of human muscle cells in terms of suppressibility of glucose oxidation by fat (Randle effect) and adaptability of fat oxidation to increasing amounts of fat (high-fat feeding adaptability) that were correlated with these same phenotypes observed in vivo of the donors (Ukropcova et al., 2005). Clearly, further study is needed to better understand the cellular changes that occur in muscle during – or in response to -- acute exercise, which could provide mechanistic basis for exercise improvements in health and disease. It stands to reason that primary human muscle cells could fill a gap in human clinical exercise trials and serve as a ‘disease in a dish’ model in which exercise mimetics (eg, electrical pulse stimulation, synthetic and naturally-occurring compounds) are utilized to dig deeper on molecular modifications, signaling pathways and mechanism-driven responses to exercise.
Effects of exercise training and calorie restriction-induced weight loss on metabolic flexibility
Exercise training induces changes in the epigenome, transcriptome and proteome to support increased storage of fuel and increased capacity for substrate utilization. In this sense, this anabolic flexibility supports improved metabolic flexibility. Exercise training can promote higher rates of fatty acid oxidation at rest and during acute exercise (van Loon et al., 1999). Exercise has the dual effect of enhancing insulin sensitivity (James et al., 1984), with the likely downstream benefits of reducing diabetes and cardiovascular disease risk. In a different context, the improved insulin sensitivity with exercise training enhances muscle glycogen storage (Sherman et al., 1981), which improves endurance exercise performance (Karlsson and Saltin, 1971). Several plausible mechanisms can explain improved insulin sensitivity and enhanced metabolic flexibility with exercise training. Increases in skeletal muscle mitochondrial biogenesis, mitochondria content and function have been reported to explain improvements in both insulin sensitivity (Coen et al., 2015a) and increased capacity for fatty acid oxidation (Jong-Yeon et al., 2002). Calorie restriction-induced weight loss also improves insulin sensitivity (Coen et al., 2015b), but in contrast to exercise, does not seem to enhance the capacity of skeletal muscle for fatty acid oxidation (Toledo and Goodpaster, 2013). Remarkably, these lack of improvements in fatty acid oxidation of skeletal muscle mitochondria correspond to the lack of response to weight loss (Coen et al., 2015a), although some studies have shown that calorie restriction increases mitochondrial content (Civitarese et al., 2007) and function (Vijgen et al., 2013).
Reducing fat mass via surgical removal (liposuction) of WAT does not produce metabolically beneficial results (Klein et al., 2004), pointing toward the need for a calorie-restriction-induced and/or an exercise-induced remodeling of WAT in order to achieve metabolic improvements within the adipose tissue. Little is known about the effects of exercise on metabolic flexibility (in terms of insulin and acute exercise bout responsiveness) and the related molecular mechanisms in WAT. Calorie-restriction-induced weight loss has a broader effect on the transcriptome in WAT compared with calorie restriction plus exercise (Lam et al., 2016). Additional studies are warranted to determine whether exercise training and weight loss may have common signatures that improve mitochondrial function, efficiency or reduce oxidative stress concomitant with enhanced metabolic flexibility.
Could metabolic flexibility be a target to prevent or treat disease?
Metabolic flexibility encompasses a variety of pathways and mechanisms. To the extent that one target could be engaged to alter fuel selection or energy expenditure, metabolic flexibility, or at least components of these, therefore, could be viable targets for therapy. Enormous efforts have been made to alter metabolic flexibility in obesity and diabetes. A key controversy in this area is whether alterations in fuel selection without concomitant increases in energy demand will prove to be therapeutic in the face of nutrient overload or obesity (see review by Muoio (Muoio, 2014). For example, increasing mitochondrial fatty acid flux and oxidation may (Bruce et al., 2009) or may not (Koves et al., 2008) improve insulin resistance. Neither strategy, however, increases energy expenditure or demand (like exercise). Simply put, while strategies to alter substrate metabolism or metabolic flexibility could impact obesity and metabolic disease in the context of nutrient overload, without a concomitant increase in energy demand, they do not represent a true exercise mimetic.
There are also several examples in oncology whereby drugs are targeted to tumor metabolism have anti-tumorigenic effects (Chaube et al., 2015). Insulin sensitizers have been shown to be reasonably effective for treatment of type 2 diabetes (Eldor et al., 2013). Drugs that affect fatty acid oxidation, including PPAR agonists (Sahebkar et al., 2014), ACC inhibitors (Bourbeau and Bartberger, 2015), have been developed to affect fuel metabolism in obesity, diabetes and heart disease. AMPK activators (AICAR, Methotrexate, Metformin) have also shown promising results in targeting metabolic flexibility to treat metabolic disease (Hardie, 2013; Zhang et al., 2009). Interestingly, some of these compounds have been developed and touted as exercise mimetics. Given that exercise profoundly affects metabolic flexibility, similar strategies to improve both metabolic flexibility and sports performance have been employed. Perhaps the most notable recent example of this has been the documented and well-publicized use of Melondrate, which has been used by some countries (not approved in the U.S.) to inhibit fatty acid oxidation through decreases in carnitine palmitoyl transferase (CPT), in order to treat cardiac dysfunction and impaired cardiac metabolism (Arduini and Zammit, 2016). However, there has been little documentation of any performance benefit using this compound, despite its clear effect on energy or fuel utilization. Any pharmacologic strategy purporting to mimic exercise would need to impact metabolic flexibility and also induce increases in energy expenditure and demand similar to exercise. This should prove to be challenging if not impossible. In summary, targeting energy metabolism without unwanted negative side effects has proven to be extremely difficult in practice.
Concluding Remarks
The broad concepts of metabolic flexibility have prompted decades of investigation into factors and mechanisms influencing energy availability and fuel selection. Much of the early work focused on understanding insulin resistance in skeletal muscle and adipose tissues as part of an overall metabolic inflexibility. More recent studies have investigated metabolic flexibility within muscle and adipose cells, and their respective roles in overall metabolic flexibility. Studies employing fasting to feeding (or insulin stimulation), rest to exercise, or exercise training interventions with adipose and muscle biopsies have revealed important mechanistic clues underpinning metabolic flexibility in humans as summarized in Figure 5. Efforts should be continued to interrogate mechanisms and potential treatments for insulin resistance and metabolic inflexibility, including the capacity for fatty acid oxidation, underlying obesity, type 2 diabetes and related conditions.
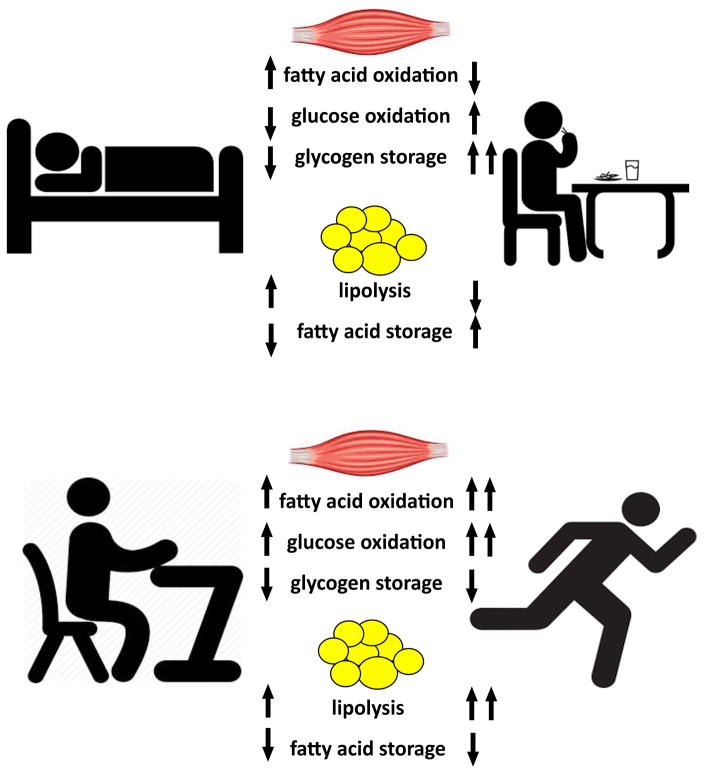
Figure 5. Summary of fuel metabolism changes within skeletal muscle and adipose tissue during periods of sleeping, fasting, feeding, rest and exercise.
Skeletal muscle switches from higher rates of fatty acid oxidation during sleeping/post-absorptive conditions to greater oxidation and storage of glucose after feeding, and reduced fatty acid oxidation. Adipose tissue shifts from higher rates of lipolysis to suppression of lipolysis and fat storage during the fasting to feeding transition. From rest to exercise, skeletal muscle increases rates of both fatty acid and glucose oxidation to support higher energy demands, while lipolysis in adipose tissue is drastically enhanced.
Acknowledgments
We express our sincere gratitude to Dr. David E. Kelley and Dr. Steven R. Smith who have profoundly shaped our thoughts about metabolic flexibility over the past 20 years. We would also like to thank the many others who have contributed to the studies and concepts highlighted in this review. Lastly, we thank Dr. Elvis Carnero for his help with the data and creating the figure on metabolic flexibility using whole room calorimetry. The authors are supported by grants from the National Institutes of Health and the American Diabetes Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aas V, Bakke SS, Feng YZ, Kase ET, Jensen J, Bajpeyi S, Thoresen GH, Rustan AC. Are cultured human myotubes far from home? Cell Tissue Res. 2013;354:671–682. doi: 10.1007/s00441-013-1655-1. [DOI] [PubMed] [Google Scholar]
- Acheson KJ, Ravussin E, Wahren J, Jequier E. Thermic effect of glucose in man. Obligatory and facultative thermogenesis. Journal of Clinical Investigation. 1984;74:1572–1580. doi: 10.1172/JCI111573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amati F, Dube JJ, Alvarez-Carnero E, Edreira MM, Chomentowski P, Coen PM, Switzer GE, Bickel PE, Stefanovic-Racic M, Toledo FG, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 2011;60:2588–2597. doi: 10.2337/db10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres R, Cadar G, Zierler K. The quantitatively minor role of carbohydrate in oxidative metabolism by skeletal muscle in intact man in the basal state. Journal of Clinical Investigation. 1956;35:671–682. doi: 10.1172/JCI103324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arduini A, Zammit VA. A tennis lesson: sharp practice in the science behind the Sharapova case. Postgrad Med J. 2016;92:429–430. doi: 10.1136/postgradmedj-2016-134124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann Med. 1995;27:435–438. doi: 10.3109/07853899709002451. [DOI] [PubMed] [Google Scholar]
- Arner P, Hellstrom L, Wahrenberg H, Bronnegard M. Beta-adrenoceptor expression in human fat cells from different regions. The Journal of clinical investigation. 1990a;86:1595–1600. doi: 10.1172/JCI114880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P, Kriegholm E, Engfeldt P, Bolinder J. Adrenergic regulation of lipolysis in situ at rest and during exercise. The Journal of clinical investigation. 1990b;85:893–898. doi: 10.1172/JCI114516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science. 2016;354:994–999. doi: 10.1126/science.aah4965. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbeau MP, Bartberger MD. Recent advances in the development of acetyl-CoA carboxylase (ACC) inhibitors for the treatment of metabolic disease. J Med Chem. 2015;58:525–536. doi: 10.1021/jm500695e. [DOI] [PubMed] [Google Scholar]
- Brehm A, Krssak M, Schmid AI, Nowotny P, Waldhausl W, Roden M. Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes. 2006;55:136–140. [PubMed] [Google Scholar]
- Brooks GA. Importance of the ‘crossover’ concept in exercise metabolism. Clinical & Experimental Pharmacology & Physiology. 1997;24:889–895. doi: 10.1111/j.1440-1681.1997.tb02712.x. [DOI] [PubMed] [Google Scholar]
- Bruce CR, Hoy AJ, Turner N, Watt MJ, Allen TL, Carpenter K, Cooney GJ, Febbraio MA, Kraegen EW. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes. 2009;58:550–558. doi: 10.2337/db08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PJ, Carlson MG, Nurjhan N. Fat metabolism in human obesity. Am J Physiol. 1994;266:E600–605. doi: 10.1152/ajpendo.1994.266.4.E600. [DOI] [PubMed] [Google Scholar]
- Chaube B, Malvi P, Singh SV, Mohammad N, Meena AS, Bhat MK. Targeting metabolic flexibility by simultaneously inhibiting respiratory complex I and lactate generation retards melanoma progression. Oncotarget. 2015;6:37281–37299. doi: 10.18632/oncotarget.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DL, Liess C, Poljak A, Xu A, Zhang J, Thoma C, Trenell M, Milner B, Jenkins AB, Chisholm DJ, et al. Phenotypic Characterization of Insulin-Resistant and Insulin-Sensitive Obesity. J Clin Endocrinol Metab. 2015;100:4082–4091. doi: 10.1210/jc.2015-2712. [DOI] [PubMed] [Google Scholar]
- Choi SM, Tucker DF, Gross DN, Easton RM, DiPilato LM, Dean AS, Monks BR, Birnbaum MJ. Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Mol Cell Biol. 2010;30:5009–5020. doi: 10.1128/MCB.00797-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E, Team CP. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen PM, Dube JJ, Amati F, Stefanovic-Racic M, Ferrell RE, Toledo FG, Goodpaster BH. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes. 2010;59:80–88. doi: 10.2337/db09-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen PM, Menshikova EV, Distefano G, Zheng D, Tanner CJ, Standley RA, Helbling NL, Dubis GS, Ritov VB, Xie H, et al. Exercise and Weight Loss Improve Muscle Mitochondrial Respiration, Lipid Partitioning, and Insulin Sensitivity After Gastric Bypass Surgery. Diabetes. 2015a;64:3737–3750. doi: 10.2337/db15-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen PM, Tanner CJ, Helbling NL, Dubis GS, Hames KC, Xie H, Eid GM, Stefanovic-Racic M, Toledo FG, Jakicic JM, et al. Clinical trial demonstrates exercise following bariatric surgery improves insulin sensitivity. The Journal of clinical investigation. 2015b;125:248–257. doi: 10.1172/JCI78016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colberg SR, Hagberg JM, McCole SD, Zmuda JM, Thompson PD, Kelley DE. Utilization of glycogen but not plasma glucose is reduced in individuals with NIDDM during mild-intensity exercise. Journal of Applied Physiology. 1996;81:2027–2033. doi: 10.1152/jappl.1996.81.5.2027. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divertie GD, Jensen MD, Miles JM. Stimulation of lipolysis in humans by physiological hypercortisolemia. Diabetes. 1991;40:1228–1232. doi: 10.2337/diab.40.10.1228. [DOI] [PubMed] [Google Scholar]
- Dube JJ, Coen PM, DiStefano G, Chacon AC, Helbling NL, Desimone ME, Stafanovic-Racic M, Hames KC, Despines AA, Toledo FG, et al. Effects of acute lipid overload on skeletal muscle insulin resistance, metabolic flexibility, and mitochondrial performance. American journal of physiology. Endocrinology and metabolism. 2014;307:E1117–1124. doi: 10.1152/ajpendo.00257.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube JJ, Sitnick MT, Schoiswohl G, Wills RC, Basantani MK, Cai L, Pulinilkunnil T, Kershaw EE. Adipose triglyceride lipase deletion from adipocytes, but not skeletal myocytes, impairs acute exercise performance in mice. American journal of physiology. Endocrinology and metabolism. 2015;308:E879–890. doi: 10.1152/ajpendo.00530.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell metabolism. 2013;17:162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Eldor R, DeFronzo RA, Abdul-Ghani M. In vivo actions of peroxisome proliferator-activated receptors: glycemic control, insulin sensitivity, and insulin secretion. Diabetes Care. 2013;36(Suppl 2):S162–174. doi: 10.2337/dcS13-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falta W, Boller R. Insularer und Insulinresistenter Diabetes. Klinische Wochenschrift. 1931;10:438–443. [Google Scholar]
- Flier JS, Kahn CR, Roth J. Receptors, antireceptor antibodies and mechanisms of insulin resistance. N Engl J Med. 1979;300:413–419. doi: 10.1056/NEJM197902223000808. [DOI] [PubMed] [Google Scholar]
- Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- Frayn KN, Coppack SW. Insulin resistance, adipose tissue and coronary heart disease. Clin Sci (Lond) 1992;82:1–8. doi: 10.1042/cs0820001. [DOI] [PubMed] [Google Scholar]
- Frayn KN, Williams CM, Arner P. Are increased plasma non-esterified fatty acid concentrations a risk marker for coronary heart disease and other chronic diseases? Clin Sci (Lond) 1996;90:243–253. doi: 10.1042/cs0900243. [DOI] [PubMed] [Google Scholar]
- Girard J. Mechanisms of action of thiazolidinediones. Diabetes Metab. 2001;27:271–278. [PubMed] [Google Scholar]
- Goodpaster B, Kelley D. In: Metabolic inflexibility and insulin resistance in skeletal muscle. In Physical Activity and Type 2 Diabetes. Hawley, Zierath, editors. Champaign: Human Kinetics; 2008. pp. 59–66. [Google Scholar]
- Goodpaster BH, Costill DL, Fink WJ, Trappe TA, Jozsi AC, Starling RD, Trappe SW. The effects of pre-exercise starch ingestion on endurance performance. Int J Sports Med. 1996;17:366–372. doi: 10.1055/s-2007-972862. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Wolfe RR, Kelley DE. Effects of obesity on substrate utilization during exercise. Obes Res. 2002;10:575–584. doi: 10.1038/oby.2002.78. [DOI] [PubMed] [Google Scholar]
- Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annual review of medicine. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. The Journal of clinical investigation. 1989;84:205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan HP, Li Y, Jensen MV, Newgard CB, Steppan CM, Lazar MA. A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat Med. 2002;8:1122–1128. doi: 10.1038/nm780. [DOI] [PubMed] [Google Scholar]
- Hansen M, Morthorst R, Larsson B, Dall R, Flyvbjerg A, Rasmussen MH, Orskov H, Kjaer M, Lange KH. No effect of growth hormone administration on substrate oxidation during exercise in young, lean men. J Physiol. 2005;567:1035–1045. doi: 10.1113/jphysiol.2005.089524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes. 2013;62:2164–2172. doi: 10.2337/db13-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell. 2014;159:738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Healy ML, Gibney J, Pentecost C, Croos P, Russell-Jones DL, Sonksen PH, Umpleby AM. Effects of high-dose growth hormone on glucose and glycerol metabolism at rest and during exercise in endurance-trained athletes. J Clin Endocrinol Metab. 2006;91:320–327. doi: 10.1210/jc.2005-0916. [DOI] [PubMed] [Google Scholar]
- Henderson GC, Fattor JA, Horning MA, Faghihnia N, Johnson ML, Mau TL, Luke-Zeitoun M, Brooks GA. Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J Physiol. 2007;584:963–981. doi: 10.1113/jphysiol.2007.137331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeks J, van Herpen NA, Mensink M, Moonen-Kornips E, van Beurden D, Hesselink MK, Schrauwen P. Prolonged fasting identifies skeletal muscle mitochondrial dysfunction as consequence rather than cause of human insulin resistance. Diabetes. 2010;59:2117–2125. doi: 10.2337/db10-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman NJ, Parker BL, Chaudhuri R, Fisher-Wellman KH, Kleinert M, Humphrey SJ, Yang P, Holliday M, Trefely S, Fazakerley DJ, et al. Global Phosphoproteomic Analysis of Human Skeletal Muscle Reveals a Network of Exercise-Regulated Kinases and AMPK Substrates. Cell metabolism. 2015;22:922–935. doi: 10.1016/j.cmet.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocrine reviews. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz JF. Fatty acid mobilization from adipose tissue during exercise. Trends Endocrinol Metab. 2003;14:386–392. doi: 10.1016/s1043-2760(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Horowitz JF, Klein S. Oxidation of nonplasma fatty acids during exercise is increased in women with abdominal obesity. [see comments]. Comment in: J Appl Physiol. 2001 Jun;90(6):2520–1. Journal of Applied Physiology. 2000;89:2276–2282. doi: 10.1152/jappl.2000.89.6.2276. [DOI] [PubMed] [Google Scholar]
- Horowitz JF, Mora-Rodriguez R, Byerley LO, Coyle EF. Substrate metabolism when subjects are fed carbohydrate during exercise. Am J Physiol. 1999;276:E828–835. doi: 10.1152/ajpendo.1999.276.5.E828. [DOI] [PubMed] [Google Scholar]
- Itani SI, Rudderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C and IkB-a. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- James DE, Kraegen EW, Chisholm DJ. Effect of exercise training on whole-body insulin sensitivity and responsiveness. J Appl Physiol. 1984;56:1217–1222. doi: 10.1152/jappl.1984.56.5.1217. [DOI] [PubMed] [Google Scholar]
- Jensen MD. Adipose tissue and fatty acid metabolism in humans. J R Soc Med. 2002;95(Suppl 42):3–7. [PMC free article] [PubMed] [Google Scholar]
- Jensen MD, Ekberg K, Landau BR. Lipid metabolism during fasting. American journal of physiology. Endocrinology and metabolism. 2001;281:E789–793. doi: 10.1152/ajpendo.2001.281.4.E789. [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE. Regulation of fat metabolism in skeletal muscle. Ann N Y Acad Sci. 2002;967:217–235. doi: 10.1111/j.1749-6632.2002.tb04278.x. [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE, Saris WH, Van Diesen R, Brouns F, Wagenmakers AJ. Effect of endogenous carbohydrate availability on oral medium-chain triglyceride oxidation during prolonged exercise. Journal of Applied Physiology. 1996;80:949–954. doi: 10.1152/jappl.1996.80.3.949. [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE, Saris WH, Wagenmakers AJ. Fat metabolism during exercise: a review--part III: effects of nutritional interventions. [Review] [97 refs] International Journal of Sports Medicine. 1998a;19:371–379. doi: 10.1055/s-2007-971932. [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE, Thielen JJ, Wagenmakers AJ, Brouns F, Saris WH. Effect of medium-chain triacylglycerol and carbohydrate ingestion during exercise on substrate utilization and subsequent cycling performance. Am J Clin Nutr. 1998b;67:397–404. doi: 10.1093/ajcn/67.3.397. [DOI] [PubMed] [Google Scholar]
- Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, Verdin EM, Kahn CR. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A. 2011;108:14608–14613. doi: 10.1073/pnas.1111308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong-Yeon K, Hickner RC, Dohm GL, Houmard JA. Long- and medium-chain fatty acid oxidation is increased in exercise-trained human skeletal muscle. Metabolism: Clinical & Experimental. 2002;51:460–464. doi: 10.1053/meta.2002.31326. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Richter EA, Wojtaszewski JF. Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. J Physiol. 2006;574:17–31. doi: 10.1113/jphysiol.2006.109942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J, Saltin B. Diet, muscle glycogen, and endurance performance. Journal of Applied Physiology. 1971;31:203–206. doi: 10.1152/jappl.1971.31.2.203. [DOI] [PubMed] [Google Scholar]
- Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441–2449. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P, Vollaard NB, Gustafsson T, Gallagher IJ, Sundberg CJ, Rankinen T, Britton SL, Bouchard C, Koch LG, Timmons JA. A transcriptional map of the impact of endurance exercise training on skeletal muscle phenotype. J Appl Physiol (1985) 2011;110:46–59. doi: 10.1152/japplphysiol.00634.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94:2349–2356. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277:E1130–1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- Kelley DE, Mokan M, Simoneau J, Mandarino LJ. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest. 1993;92:91–98. doi: 10.1172/JCI116603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Mandarino LJ. Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus. Journal of Clinical Investigation. 1990;86:1999–2007. doi: 10.1172/JCI114935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. Journal of Clinical Investigation. 1993;92:91–98. doi: 10.1172/JCI116603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350:2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- Kohler P. The strategies of energy conservation in helminths. Mol Biochem Parasitol. 1985;17:1–18. doi: 10.1016/0166-6851(85)90124-0. [DOI] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell metabolism. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Lam YY, Ghosh S, Civitarese AE, Ravussin E. Six-month Calorie Restriction in Overweight Individuals Elicits Transcriptomic Response in Subcutaneous Adipose Tissue That is Distinct From Effects of Energy Deficit. J Gerontol A Biol Sci Med Sci. 2016;71:1258–1265. doi: 10.1093/gerona/glv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, Jurczak MJ, Zhang D, Woo DK, Shadel GS, Ladiges W, et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell metabolism. 2010;12:668–674. doi: 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Mansueto G, Armani A, Viscomi C, D’Orsi L, De Cegli R, Polishchuk EV, Lamperti C, Di Meo I, Romanello V, Marchet S, et al. Transcription Factor EB Controls Metabolic Flexibility during Exercise. Cell metabolism. 2017;25:182–196. doi: 10.1016/j.cmet.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR. Oscillatory insulin secretion: a variable phenotypic marker. Diabet Med. 1996;13:S53–58. [PubMed] [Google Scholar]
- Mauriege P, Galitzky J, Berlan M, Lafontan M. Heterogeneous distribution of beta and alpha-2 adrenoceptor binding sites in human fat cells from various fat deposits: functional consequences. Eur J Clin Invest. 1987;17:156–165. doi: 10.1111/j.1365-2362.1987.tb02395.x. [DOI] [PubMed] [Google Scholar]
- McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- McLean CS, Mielke C, Cordova JM, Langlais PR, Bowen B, Miranda D, Coletta DK, Mandarino LJ. Gene and MicroRNA Expression Responses to Exercise; Relationship with Insulin Sensitivity. PLoS One. 2015;10:e0127089. doi: 10.1371/journal.pone.0127089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray RG, Forsythe WA, Mar MH, Hardy CJ. Exercise intensity-related responses of beta-endorphin and catecholamines. Med Sci Sports Exerc. 1987;19:570–574. [PubMed] [Google Scholar]
- McQuaid SE, Hodson L, Neville MJ, Dennis AL, Cheeseman J, Humphreys SM, Ruge T, Gilbert M, Fielding BA, Frayn KN, et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes. 2011;60:47–55. doi: 10.2337/db10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. The Journal of clinical investigation. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio DM. Metabolic inflexibility: when mitochondrial indecision leads to metabolic gridlock. Cell. 2014;159:1253–1262. doi: 10.1016/j.cell.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell metabolism. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Ng JM, Azuma K, Kelley C, Pencek R, Radikova Z, Laymon C, Price J, Goodpaster BH, Kelley DE. PET imaging reveals distinctive roles for different regional adipose tissue depots in systemic glucose metabolism in nonobese humans. American journal of physiology. Endocrinology and metabolism. 2012;303:E1134–1141. doi: 10.1152/ajpendo.00282.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. The Journal of clinical investigation. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurjhan N, Campbell PJ, Kennedy FP, Miles JM, Gerich JE. Insulin dose-response characteristics for suppression of glycerol release and conversion to glucose in humans. Diabetes. 1986;35:1326–1331. doi: 10.2337/diab.35.12.1326. [DOI] [PubMed] [Google Scholar]
- O’Rahilly S, Turner RC, Matthews DR. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N Engl J Med. 1988;318:1225–1230. doi: 10.1056/NEJM198805123181902. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D. In: Plasticity of Muscle. Gruyter d., editor. Berlin: 1980. [Google Scholar]
- Phielix E, Meex R, Ouwens DM, Sparks L, Hoeks J, Schaart G, Moonen-Kornips E, Hesselink MK, Schrauwen P. High oxidative capacity due to chronic exercise training attenuates lipid-induced insulin resistance. Diabetes. 2012;61:2472–2478. doi: 10.2337/db11-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Rasmussen M, Zierath JR, Barres R. Dynamic epigenetic responses to muscle contraction. Drug discovery today. 2014;19:1010–1014. doi: 10.1016/j.drudis.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC, Trappe S. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol (1985) 2012;112:1625–1636. doi: 10.1152/japplphysiol.00435.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Reitman ML, Gavrilova O. A-ZIP/F-1 mice lacking white fat: a model for understanding lipoatrophic diabetes. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2000;24:S11–14. doi: 10.1038/sj.ijo.0801493. [DOI] [PubMed] [Google Scholar]
- Reshef L, Olswang Y, Cassuto H, Blum B, Croniger CM, Kalhan SC, Tilghman SM, Hanson RW. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem. 2003;278:30413–30416. doi: 10.1074/jbc.R300017200. [DOI] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol Endocrinol Metab. 1993;265:E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- Ronn T, Volkov P, Davegardh C, Dayeh T, Hall E, Olsson AH, Nilsson E, Tornberg A, Dekker Nitert M, Eriksson KF, et al. A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genet. 2013;9:e1003572. doi: 10.1371/journal.pgen.1003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronn T, Volkov P, Tornberg A, Elgzyri T, Hansson O, Eriksson KF, Groop L, Ling C. Extensive changes in the transcriptional profile of human adipose tissue including genes involved in oxidative phosphorylation after a 6-month exercise intervention. Acta Physiol (Oxf) 2014;211:188–200. doi: 10.1111/apha.12247. [DOI] [PubMed] [Google Scholar]
- Sahebkar A, Chew GT, Watts GF. New peroxisome proliferator-activated receptor agonists: potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease. Expert Opin Pharmacother. 2014;15:493–503. doi: 10.1517/14656566.2014.876992. [DOI] [PubMed] [Google Scholar]
- Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RS, Shuman WP, Larson V, Cain KC, Fellingham GW, Beard JC, Kahn SE, Stratton JR, Cerqueira MD, Abrass IB. The effect of intensive endurance exercise training on body fat distribution in young and older men. Metabolism. 1991;40:545–551. doi: 10.1016/0026-0495(91)90239-s. [DOI] [PubMed] [Google Scholar]
- Sherman WM, Costill DL, Fink WJ, Miller JM. Effect of exercise-diet manipulation on muscle glycogen and its subsequent utilization during performance. International Journal of Sports Medicine. 1981;2:114–118. doi: 10.1055/s-2008-1034594. [DOI] [PubMed] [Google Scholar]
- Shulman GI. Unraveling the cellular mechanism of insulin resistance in humans: new insights from magnetic resonance spectroscopy. Physiology. 2004;19:183–190. doi: 10.1152/physiol.00007.2004. [DOI] [PubMed] [Google Scholar]
- Sparks LM, Ukropcova B, Smith J, Pasarica M, Hymel D, Xie H, Bray GA, Miles JM, Smith SR. Relation of adipose tissue to metabolic flexibility. Diabetes Res Clin Pract. 2009;83:32–43. doi: 10.1016/j.diabres.2008.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallknecht B, Dela F, Helge JW. Are blood flow and lipolysis in subcutaneous adipose tissue influenced by contractions in adjacent muscles in humans? American journal of physiology. Endocrinology and metabolism. 2007;292:E394–399. doi: 10.1152/ajpendo.00215.2006. [DOI] [PubMed] [Google Scholar]
- Stallknecht B, Lorentsen J, Enevoldsen LH, Bulow J, Biering-Sorensen F, Galbo H, Kjaer M. Role of the sympathoadrenergic system in adipose tissue metabolism during exercise in humans. J Physiol. 2001;536:283–294. doi: 10.1111/j.1469-7793.2001.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI, Middelbeek RJ, Goodyear LJ. Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations. Diabetes. 2015;64:2361–2368. doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich V, de Glisezinski I, Berlan M, Bulow J, Galitzky J, Harant I, Suljkovicova H, Lafontan M, Riviere D, Crampes F. Adipose tissue lipolysis is increased during a repeated bout of aerobic exercise. J Appl Physiol (1985) 2000;88:1277–1283. doi: 10.1152/jappl.2000.88.4.1277. [DOI] [PubMed] [Google Scholar]
- Thomas EL, Brynes AE, McCarthy J, Goldstone AP, Hajnal JV, Saeed N, Frost G, Bell JD. Preferential loss of visceral fat following aerobic exercise, measured by magnetic resonance imaging. Lipids. 2000;35:769–776. doi: 10.1007/s11745-000-0584-0. [DOI] [PubMed] [Google Scholar]
- Toledo FG, Goodpaster BH. The role of weight loss and exercise in correcting skeletal muscle mitochondrial abnormalities in obesity, diabetes and aging. Mol Cell Endocrinol. 2013;379:30–34. doi: 10.1016/j.mce.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee-Young RS, Babb JR, Meikle PJ, Lancaster GI, Henstridge DC, et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia. 2013;56:1638–1648. doi: 10.1007/s00125-013-2913-1. [DOI] [PubMed] [Google Scholar]
- Ukropcova B, McNeil M, Sereda O, de Jonge L, Xie H, Bray GA, Smith SR. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. The Journal of clinical investigation. 2005;115:1934–1941. doi: 10.1172/JCI24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukropcova B, Sereda O, de Jonge L, Bogacka I, Nguyen T, Xie H, Bray GA, Smith SR. Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes. 2007;56:720–727. doi: 10.2337/db06-0521. [DOI] [PubMed] [Google Scholar]
- van Loon LJ, Jeukendrup AE, Saris WH, Wagenmakers AJ. Effect of training status on fuel selection during submaximal exercise with glucose ingestion. Journal of Applied Physiology. 1999;87:1413–1420. doi: 10.1152/jappl.1999.87.4.1413. [DOI] [PubMed] [Google Scholar]
- Vijgen GH, Bouvy ND, Hoeks J, Wijers S, Schrauwen P, van Marken Lichtenbelt WD. Impaired skeletal muscle mitochondrial function in morbidly obese patients is normalized one year after bariatric surgery. Surg Obes Relat Dis. 2013;9:936–941. doi: 10.1016/j.soard.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- Yu XX, Lewin DA, Forrest W, Adams SH. Cold elicits the simultaneous induction of fatty acid synthesis and beta-oxidation in murine brown adipose tissue: prediction from differential gene expression and confirmation in vivo. FASEB J. 2002;16:155–168. doi: 10.1096/fj.01-0568com. [DOI] [PubMed] [Google Scholar]
- Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell metabolism. 2009;9:407–416. doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]