Abstract
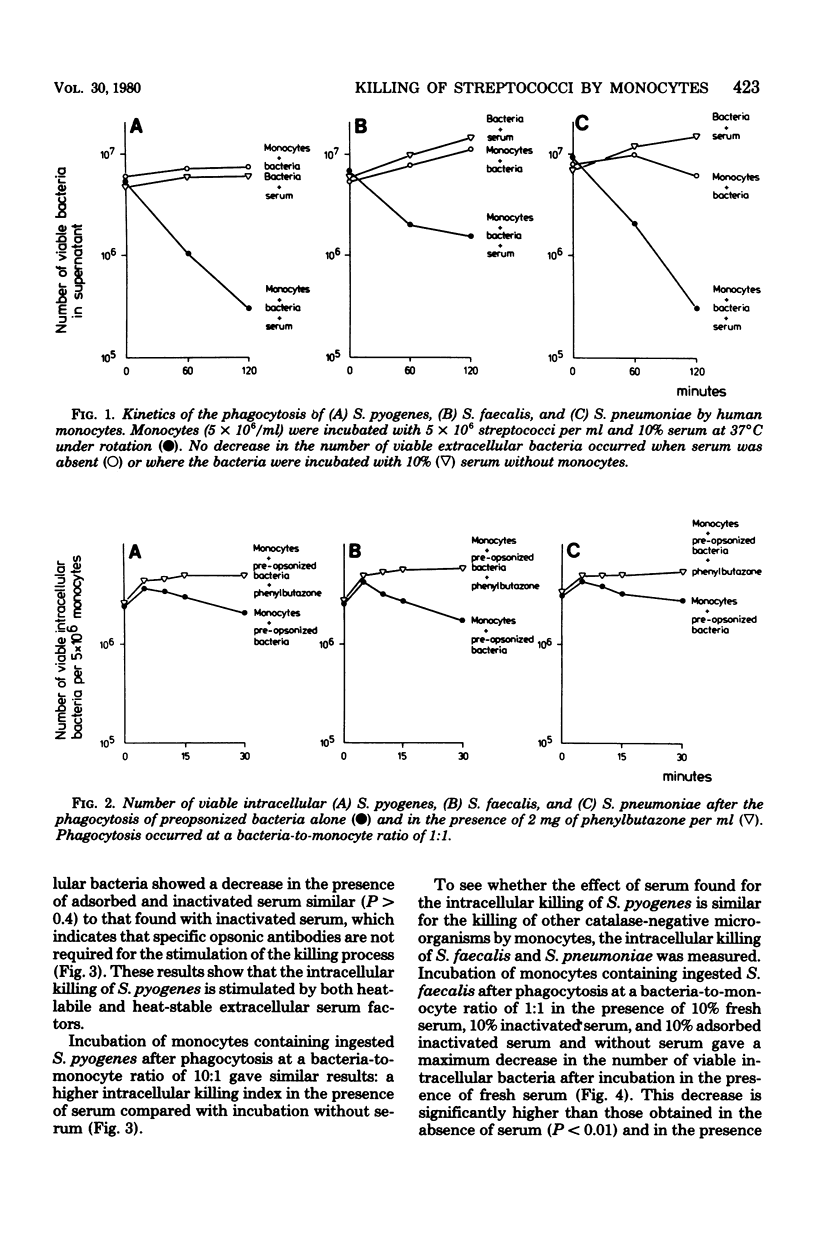
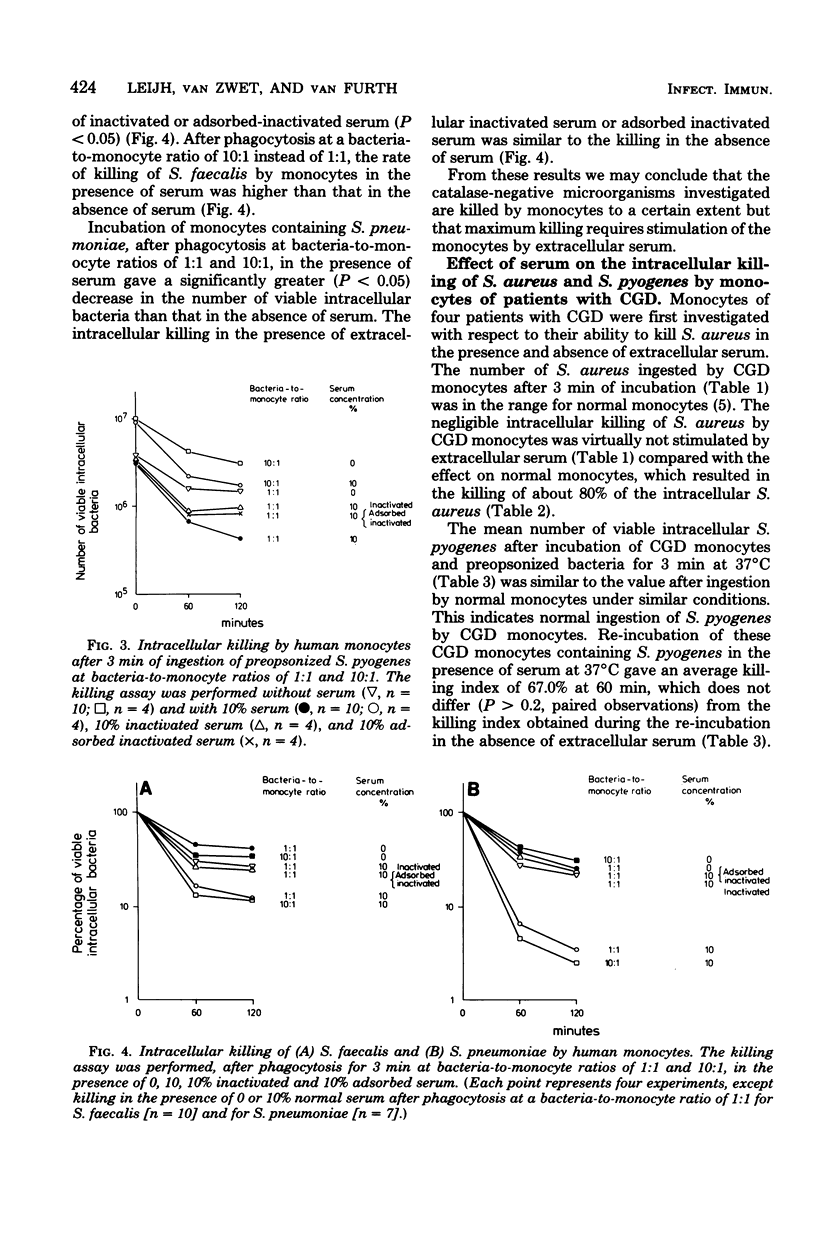
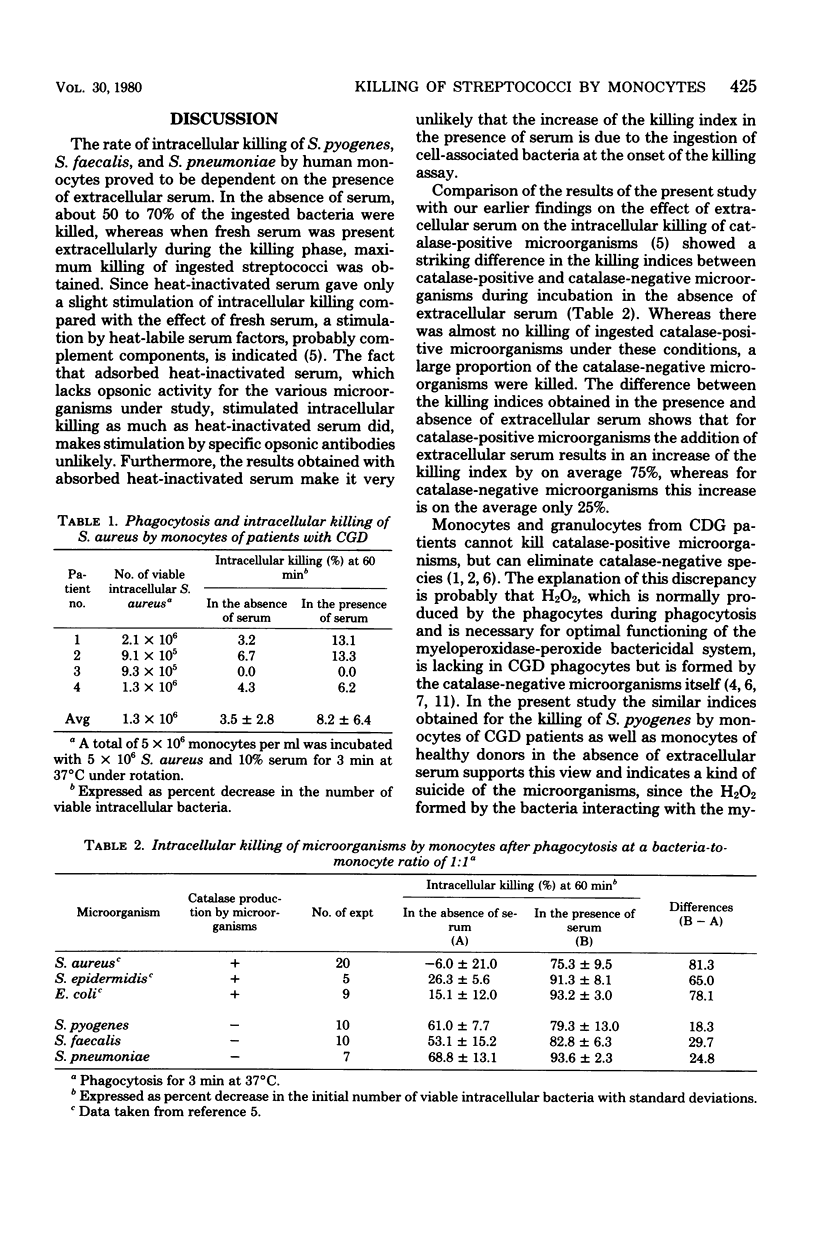
This study shows that the intracellular killing of Streptococcus pyogenes, Streptococcus faecalis, and Streptococcus pneumoniae by human monocytes is stimulated by the extracellular presence of both heat-stable and heat-labile serum factors. A similar kind of stimulation of monocytes has been described in respect of catalase-positive microorganisms. However, killing of these bacteria is negligible in the absence of extracellular serum factors, whereas a large proportion of the ingested catalase-negative bacteria are killed in the absence of such extracellular stimuli. Monocytes from patients with chronic granulomatous disease, which are unable to kill Staphylococcus aureus even in the presence of extracellular serum, killed S. pyogenes equally effectively whether serum was present or absent. This index proved to be the same as that for killing by monocytes of healthy subjects in the absence of serum. Taken together, these results indicate that catalase-negative microorganisms possess some kind of suicide mechanism that leads to the death of these bacteria after their ingestion by monocytes in the absence of an extracellular stimulus. Furthermore, the mechanism by which extracellular serum stimulates intracellular killing probably involves enzymes of the O2-dependent bactericidal mechanisms of the monocytes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E. L., Laxdal T., Quie P. G. Studies of polymorphonuclear leukocytes from patients with chronic granulomatous disease of childhood: bactericidal capacity for streptococci. Pediatrics. 1968 Mar;41(3):591–599. [PubMed] [Google Scholar]
- Klebanoff S. J., White L. R. Iodination defect in the leukocytes of a patient with chronic granulomatous disease of childhood. N Engl J Med. 1969 Feb 27;280(9):460–466. doi: 10.1056/NEJM196902272800902. [DOI] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Zwet T. L., Daha M. R., van Furth R. Requirement of extracellular complement and immunoglobulin for intracellular killing of micro-organisms by human monocytes. J Clin Invest. 1979 Apr;63(4):772–784. doi: 10.1172/JCI109362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Hook E. W. Leukocyte bactericidal activity in chronic granulomatous disease: correlation of bacterial hydrogen peroxide production and susceptibility to intracellular killing. J Bacteriol. 1969 Oct;100(1):531–532. doi: 10.1128/jb.100.1.531-532.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J., Bernheimer H. P. Role of peroxide in phagocytic killing of pneumococci. Infect Immun. 1974 Jan;9(1):48–52. doi: 10.1128/iai.9.1.48-52.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., White J. G., Clawson C. C., Holmes B. M. Effects of phorbol myristate acetate on the metabolism and ultrastructure of neutrophils in chronic granulomatous disease. J Clin Invest. 1974 Jul;54(1):83–90. doi: 10.1172/JCI107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Stossel T. P. Myeloperoxidase-mediated iodination by granulocytes. Intracellular site of operation and some regulating factors. J Clin Invest. 1974 May;53(5):1207–1215. doi: 10.1172/JCI107667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohet S. B., Pitt J., Baehner R. L., Poplack D. G. Lipid peroxidation in the killing of phagocytized pneumococci. Infect Immun. 1974 Dec;10(6):1321–1328. doi: 10.1128/iai.10.6.1321-1328.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg C. O. Influence of therapeutic concentrations of phenylbutazone on granulocyte function. Acta Pathol Microbiol Scand B. 1975 Apr;83(2):100–102. doi: 10.1111/j.1699-0463.1975.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Sbarra A. J. Effect of phenylbutazone on phagocytosis and intracellular killing by guinea pig polymorphonuclear leukocytes. J Bacteriol. 1968 Dec;96(6):1982–1990. doi: 10.1128/jb.96.6.1982-1990.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik M. L., Weening R. S., Roos D. Phenylglyoxal is not a selective inhibitor of phagocytosis. J Cell Sci. 1979 Aug;38:331–343. doi: 10.1242/jcs.38.1.331. [DOI] [PubMed] [Google Scholar]


