Abstract
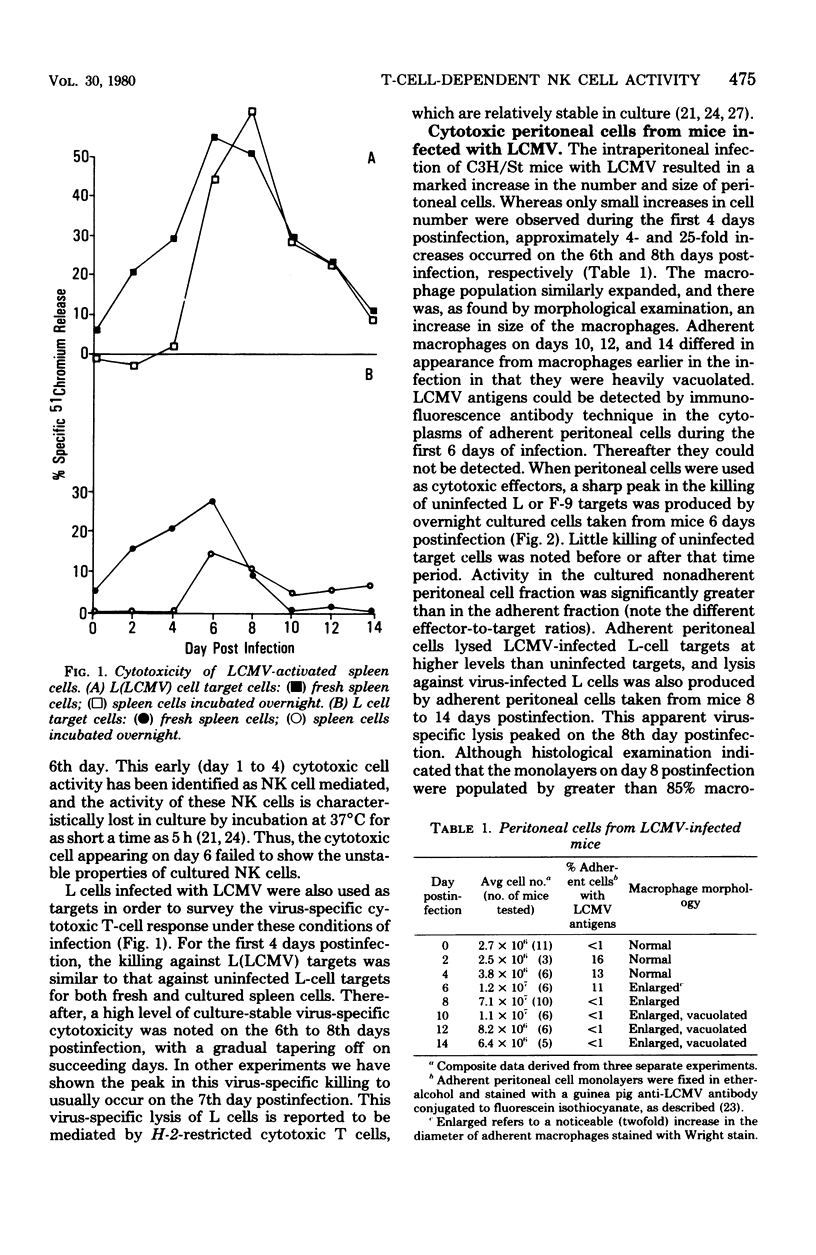
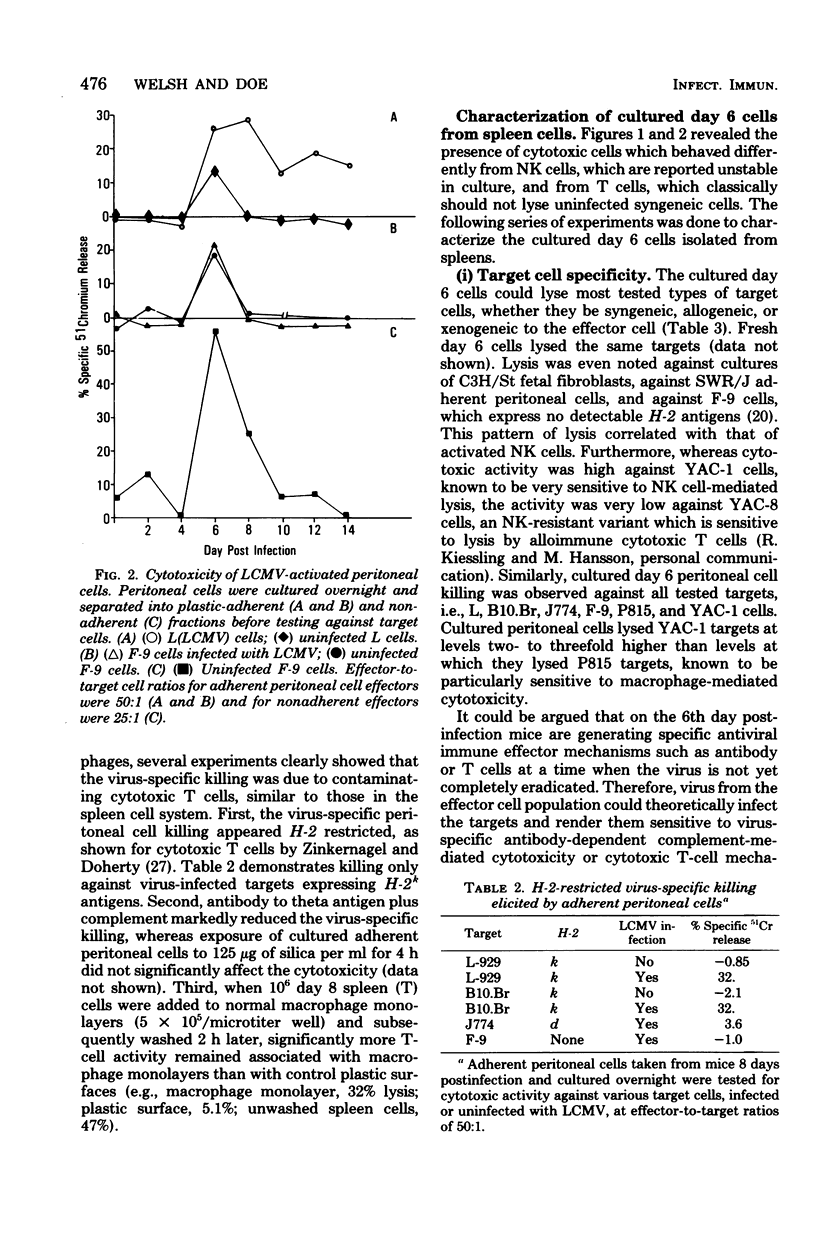
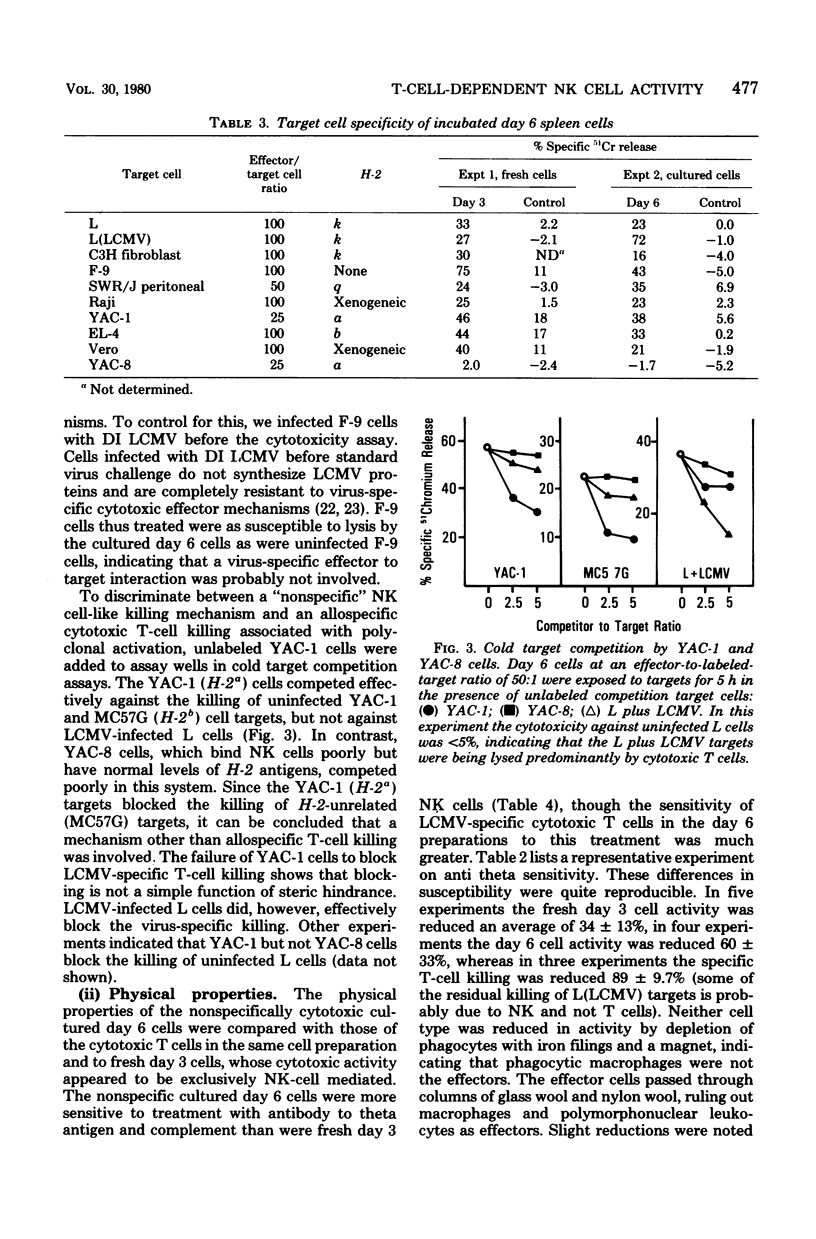
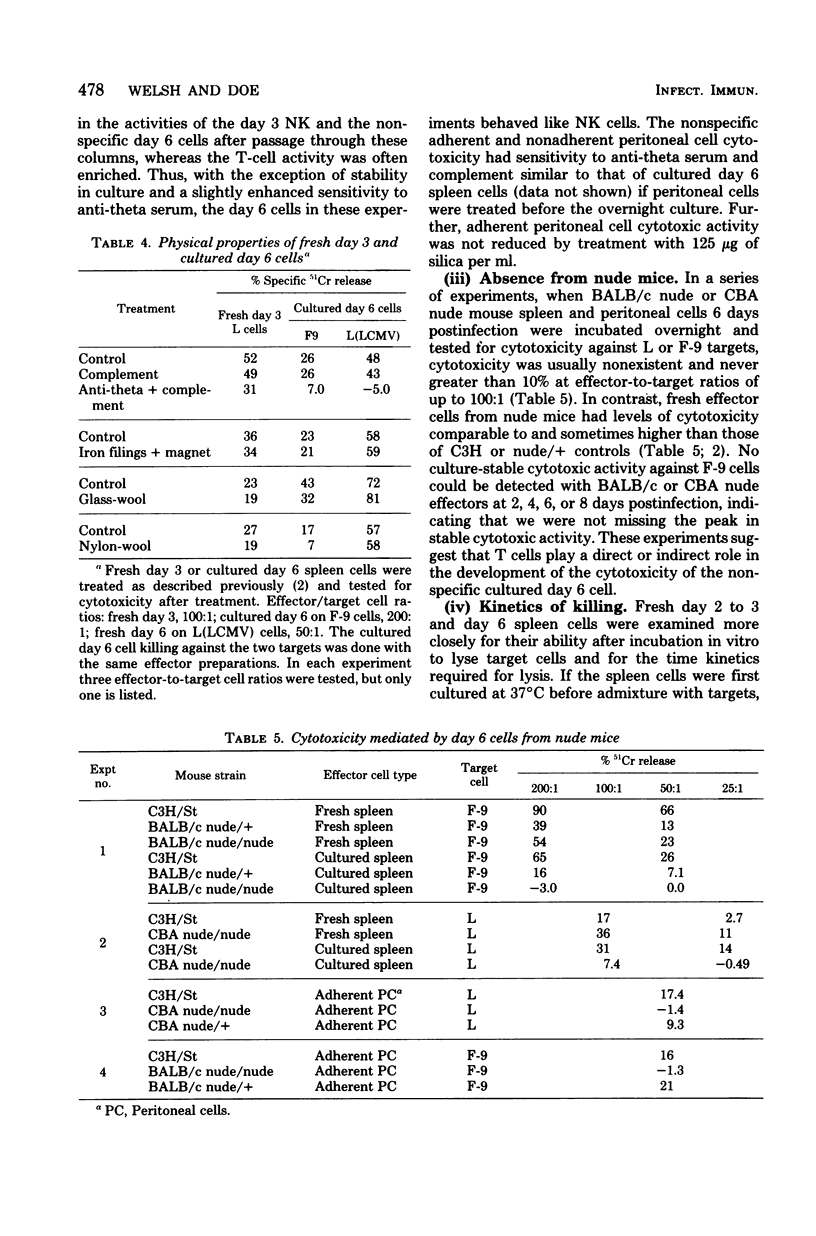
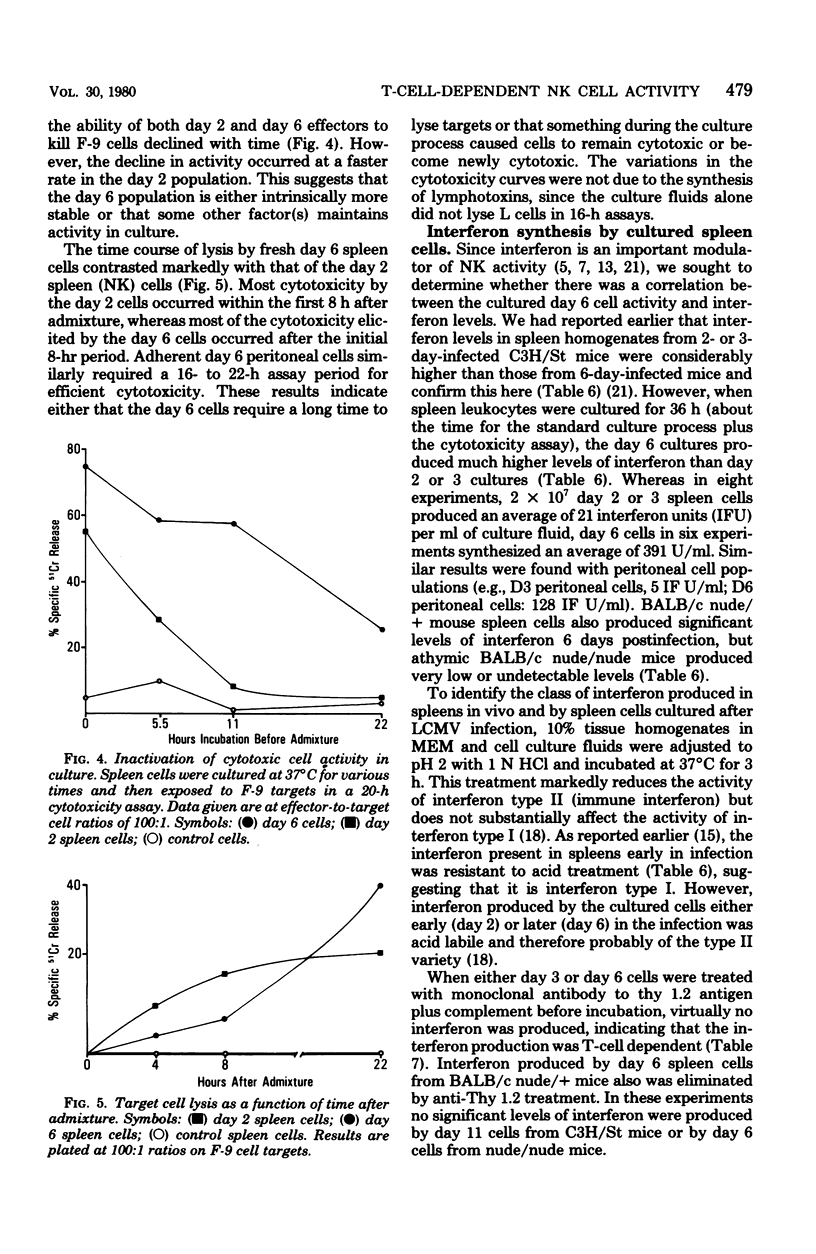
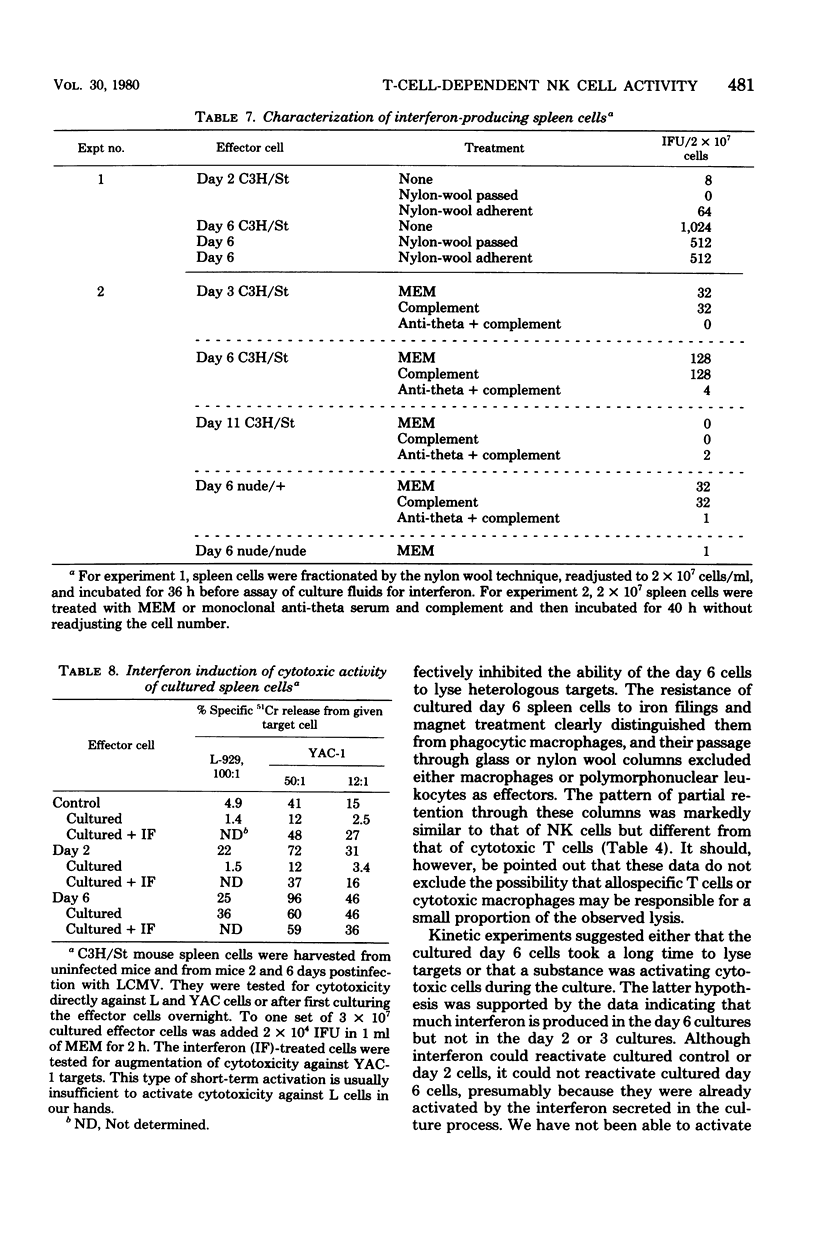
The characteristics and specificities of spleen and peritoneal cytotoxic cells generated during lymphocytic choriomeningitis virus (LCMV) infection of C3H/St mice were examined. Activated natural killer (NK) cell activity was identified in fresh leukocyte populations from the 2nd to 8th days postinfection, whereas virus-specific cytotoxic T-cell activity was detected from the 6th to 14th days. When leukocytes were cultured overnight at 37 degrees C before assay, T-cell activity was still observed, but nonspecific activated NK cell-like cytotoxicity was only detected on the 6th and to a lesser degree the 8th day postinfection. Overnight culture of leukocytes taken earlier in the infection eliminated their NK cell activity. Similar activities were seen with spleen cell, plastic-adherent peritoneal cell, and nonadherent peritoneal cell populations. The virus-specific cytotoxicity observed with adherent peritoneal cells was due to contamination with cytotoxic T cells, as shown by H-2-restricted cytotoxicity and sensitivity to anti-theta antibody and complement. The nonspecific cultured day 6 effector cell from either the spleen or peritoneum displayed killing specificities and other physical properties identical to those of activated NK cells, but had sensitivities to anti-theta antibody and complement intermediate between activated day 3 NK cells and cytotoxic T cells. Culture stable NK-like cells were not found in athymic nude mice, suggesting a T-cell-dependent mechanism. Whereas LCMV spleen homogenates contained 10-fold-higher levels of interferon at day 2 than at day 6 postinfection, substantially more (nearly 20-fold) interferon was made in cultures of day 6 cells than day 2 cells. Spleen interferon was predominantly type I, whereas the culture interferon was predominantly type II, as shown by acid lability studies. Significant levels of interferon were produced by nylon-wool-passed day 6 spleen cells, and virtually all interferon production was eliminated by treatment of either day 2 or day 6 cells with antibody to theta antigen and complement, suggesting that T cells produced the interferon in vitro. Furthermore, athymic nude mice had no culture-stable NK cells 6 days postinfection, and spleen cells from them failed to produce significant levels of interferon in vitro. Addition of interferon (type I, fibroblast) to cultured C3H spleen cells affect the already elevated levels of cytotoxicity in day 6 cultures, suggesting that the NK cells in the day 6 culture were already activated. Our results suggest that T cells responding to LCMV infection secrete interferon type II which causes the continued activation of NK cells in culture. The resulting population of activated NK cells therefore appears to be relatively stable in culture and to express more theta antigen because of this T-cell dependence. Although one could mistakenly or allospecific cytotoxic T cells or cytotoxic macrophages, more careful examination shows that they are most likely activated NK cells...
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V., Mims C. A. Macrophage activation in mice infected with ectromelia or lymphocytic choriomeningitis viruses. Aust J Exp Biol Med Sci. 1973 Jun;51(3):393–398. doi: 10.1038/icb.1973.35. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Heinbaugh J. A., Holden H. T., Herberman R. B. Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J Immunol. 1979 Jan;122(1):175–181. [PubMed] [Google Scholar]
- Doe W. F., Henson P. M. Macrophage stimulation by bacterial lipopolysaccharides. I. Cytolytic effect on tumor target cells. J Exp Med. 1978 Aug 1;148(2):544–556. doi: 10.1084/jem.148.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. B., Kreth H. W., Herzenberg L. A. Fluorescence-activated cell sorting of human T and B lymphocytes. II. Identification of the cell type responsible for interferon production and cell proliferation in response to mitogens. Cell Immunol. 1974 Jun;12(3):407–421. doi: 10.1016/0008-8749(74)90097-5. [DOI] [PubMed] [Google Scholar]
- Gidlund M., Orn A., Wigzell H., Senik A., Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978 Jun 29;273(5665):759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975 Aug 15;16(2):230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T. Low density of Thy 1 antigen on mouse effector cells mediating natural cytotoxicity against tumor cells. J Immunol. 1978 Jul;121(1):304–309. [PubMed] [Google Scholar]
- Kiessling R. W., Welsh R. M., Jr Killing of normal cells by activated mouse natural killer cells: evidence for two patterns of genetic regulation of lysis. Int J Cancer. 1980 May 15;25(5):611–615. doi: 10.1002/ijc.2910250510. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Kumar V., Ben-Ezra J., Bennett M., Sonnenfeld G. Natural killer cells in mice treated with 89strontium: normal target-binding cell numbers but inability to kill even after interferon administration. J Immunol. 1979 Oct;123(4):1832–1838. [PubMed] [Google Scholar]
- Kärre K., Seeley J. K. Cytotoxic Thy 1,2-positive blasts with NK-like target selectivity in murine mixed lymphocyte cultures. J Immunol. 1979 Oct;123(4):1511–1518. [PubMed] [Google Scholar]
- Lopez L. R., Vatter A. E., Talmage D. W. The requirement of viable thymocytes for species-specific attachment to and release from macrophages. J Immunol. 1977 Nov;119(5):1668–1673. [PubMed] [Google Scholar]
- Merigan T. C., Oldstone M. B., Welsh R. M. Interferon production during lymphocytic choriomeningitis virus infection of nude and normal mice. Nature. 1977 Jul 7;268(5615):67–68. doi: 10.1038/268067a0. [DOI] [PubMed] [Google Scholar]
- Nunn M. E., Herberman R. B., Holden H. T. Natural cell-mediated cytotoxicity in mice against non-lymphoid tumor cells and some normal cells. Int J Cancer. 1977 Sep 15;20(3):381–387. doi: 10.1002/ijc.2910200309. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Zinkernagel R. M., Oldstone M. B. Immune response in humans after vaccination with vaccinia virus: generation of a virus-specific cytotoxic activity by human peripheral lymphocytes. J Exp Med. 1977 Oct 1;146(4):949–969. doi: 10.1084/jem.146.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvin S. B., Youngner J. S., Lederer W. H. Migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. Infect Immun. 1973 Jan;7(1):68–75. doi: 10.1128/iai.7.1.68-75.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Secretory function of mononuclear phagocytes: a review. Am J Pathol. 1976 May;83(2):396–418. [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Artzt K., Bennett D., Boyse E. A., Jacob F. Structural similarities between a product of the T/t-locus isolated from sperm and teratoma cells, and H-2 antigens isolated from splenocytes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3215–3219. doi: 10.1073/pnas.72.8.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Buchmeier M. J. Protein analysis of defective interfering lymphocytic choriomeningitis virus and persistently infected cells. Virology. 1979 Jul 30;96(2):503–515. doi: 10.1016/0042-6822(79)90107-7. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J Exp Med. 1978 Jul 1;148(1):163–181. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Zinkernagel R. M., Hallenbeck L. A. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. II. "Specificities" of the natural killer cells. J Immunol. 1979 Feb;122(2):475–481. [PubMed] [Google Scholar]
- Welsh R. M., Jr, Zinkernagel R. M. Heterospecific cytotoxic cell activity induced during the first three days of acute lymphocytic choriomeningitis virus infection in mice. Nature. 1977 Aug 18;268(5621):646–648. doi: 10.1038/268646a0. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Oldstone M. B. Inhibition of immunologic injury of cultured cells infected with lymphocytic choriomeningitis virus: role of defective interfering virus in regulating viral antigenic expression. J Exp Med. 1977 Jun 1;145(6):1449–1468. doi: 10.1084/jem.145.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietzerbin J., Stephanos S., Falcoff R., Falcoff E. Some properties of interferons induced by stimulants of T and B lymphocytes. Tex Rep Biol Med. 1977;35:205–211. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. H-2 compatability requirement for T-cell-mediated lysis of target cells infected with lymphocytic choriomeningitis virus. Different cytotoxic T-cell specificities are associated with structures coded for in H-2K or H-2D;. J Exp Med. 1975 Jun 1;141(6):1427–1436. doi: 10.1084/jem.141.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]


