Abstract
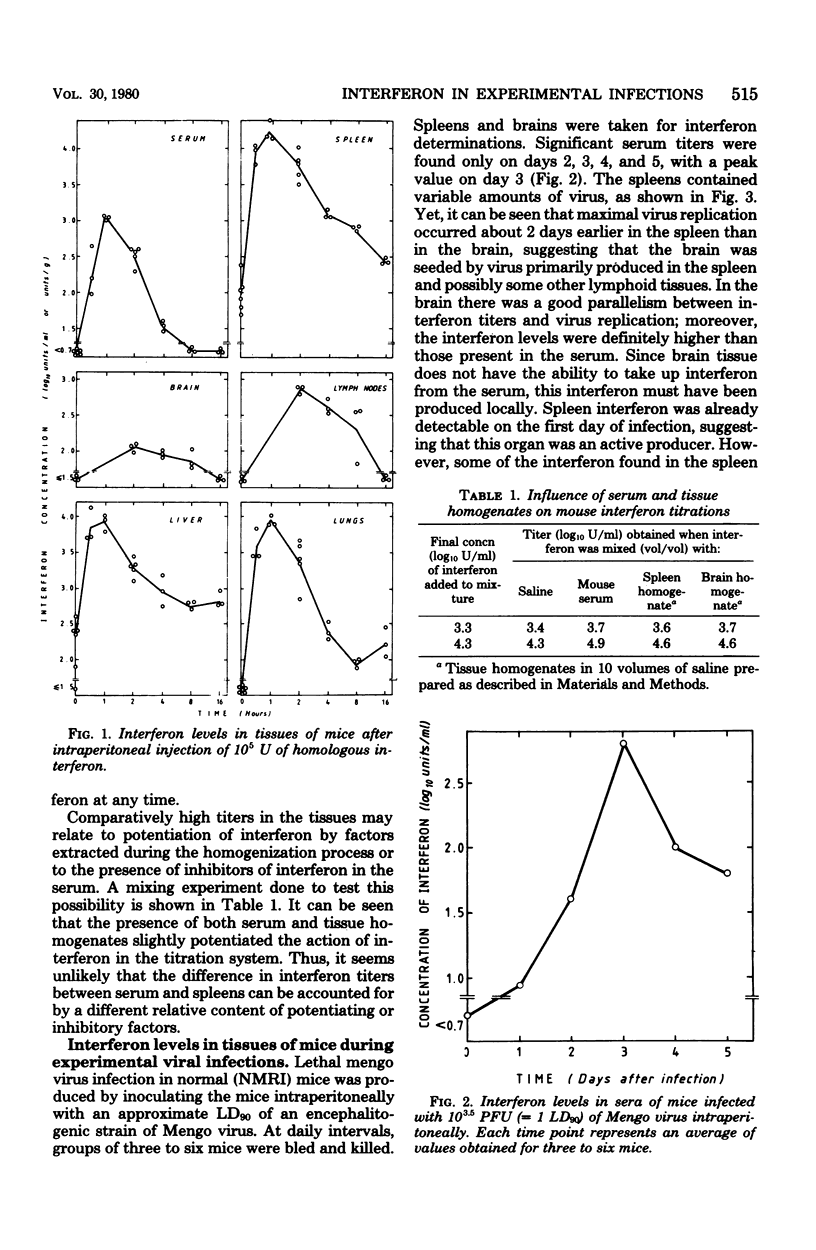
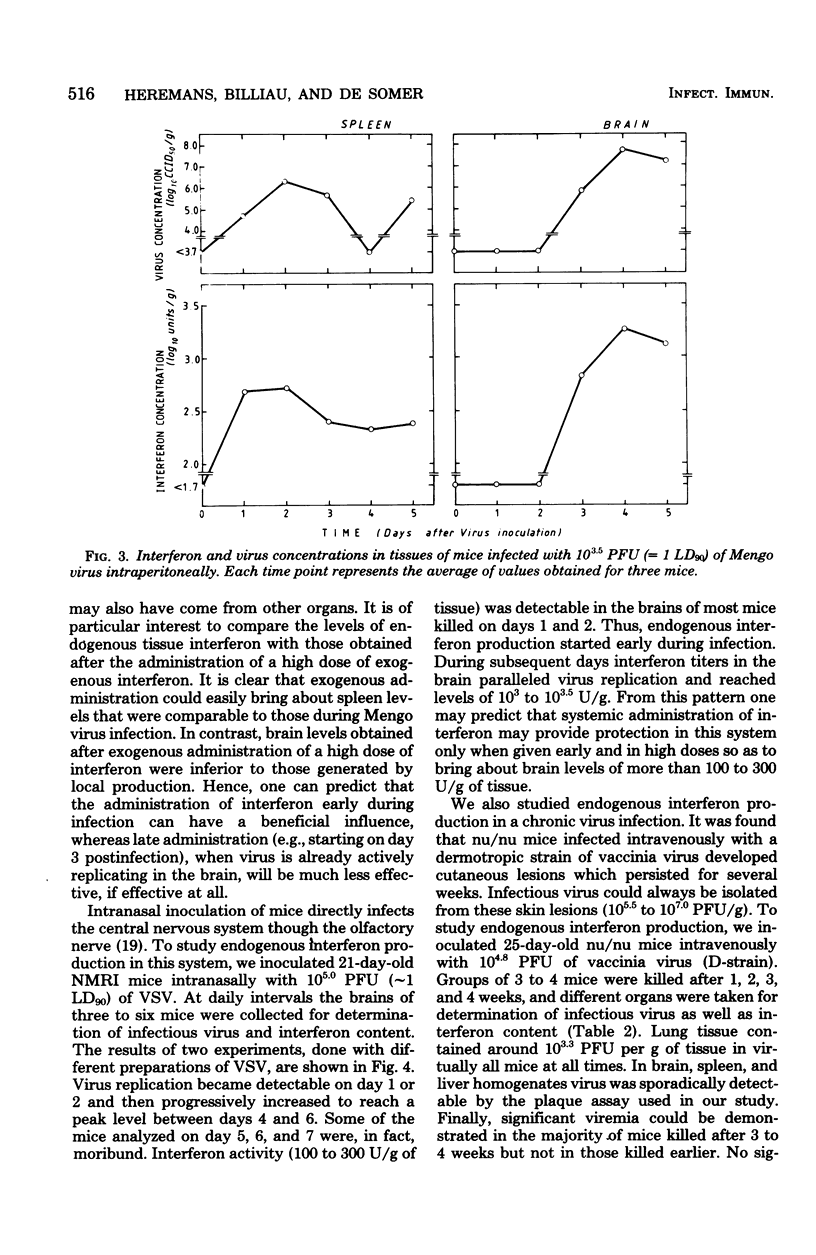
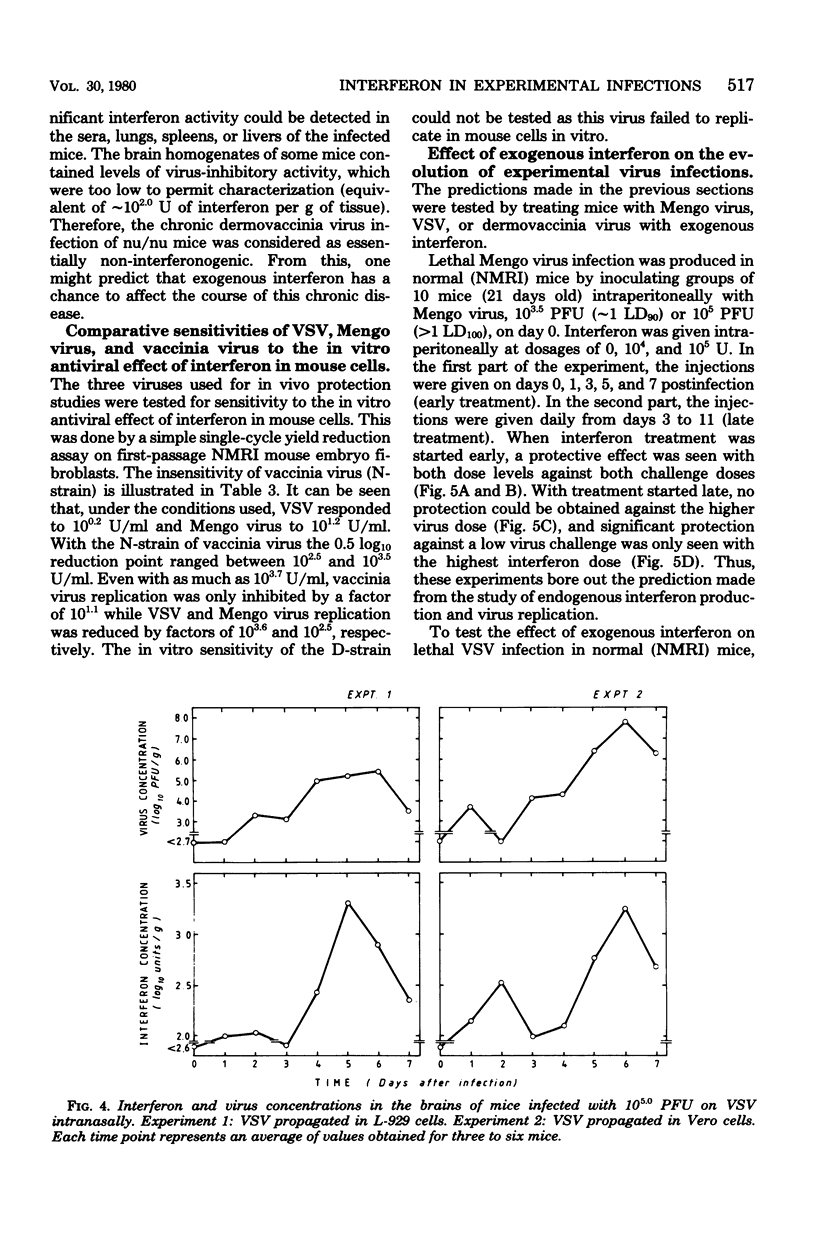
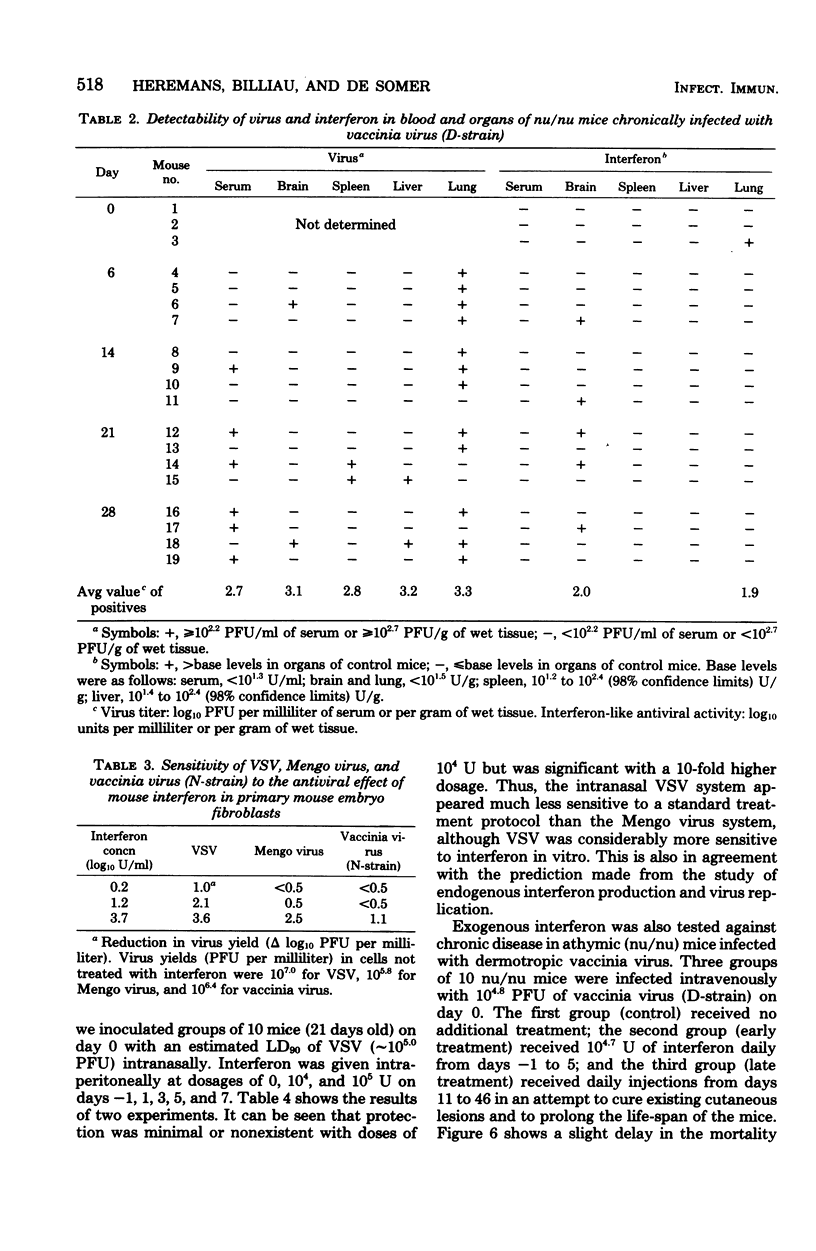
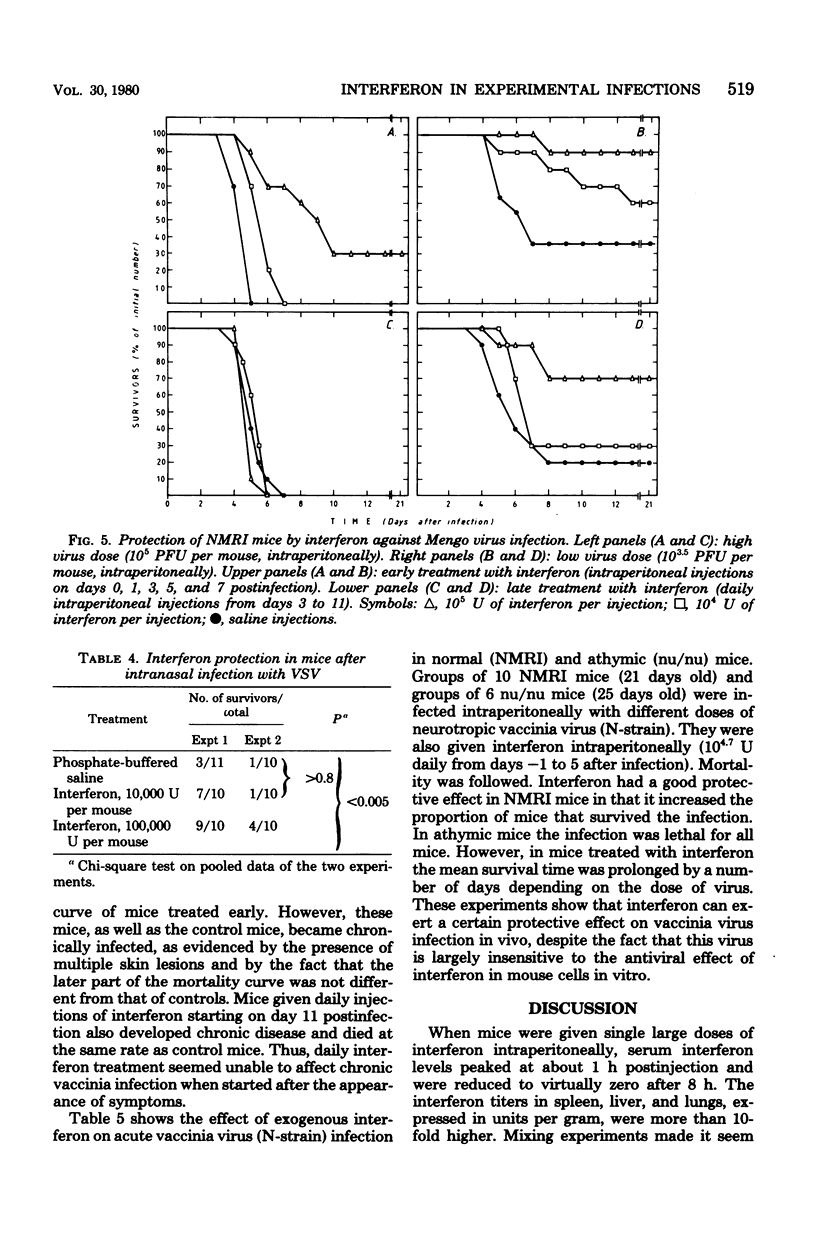
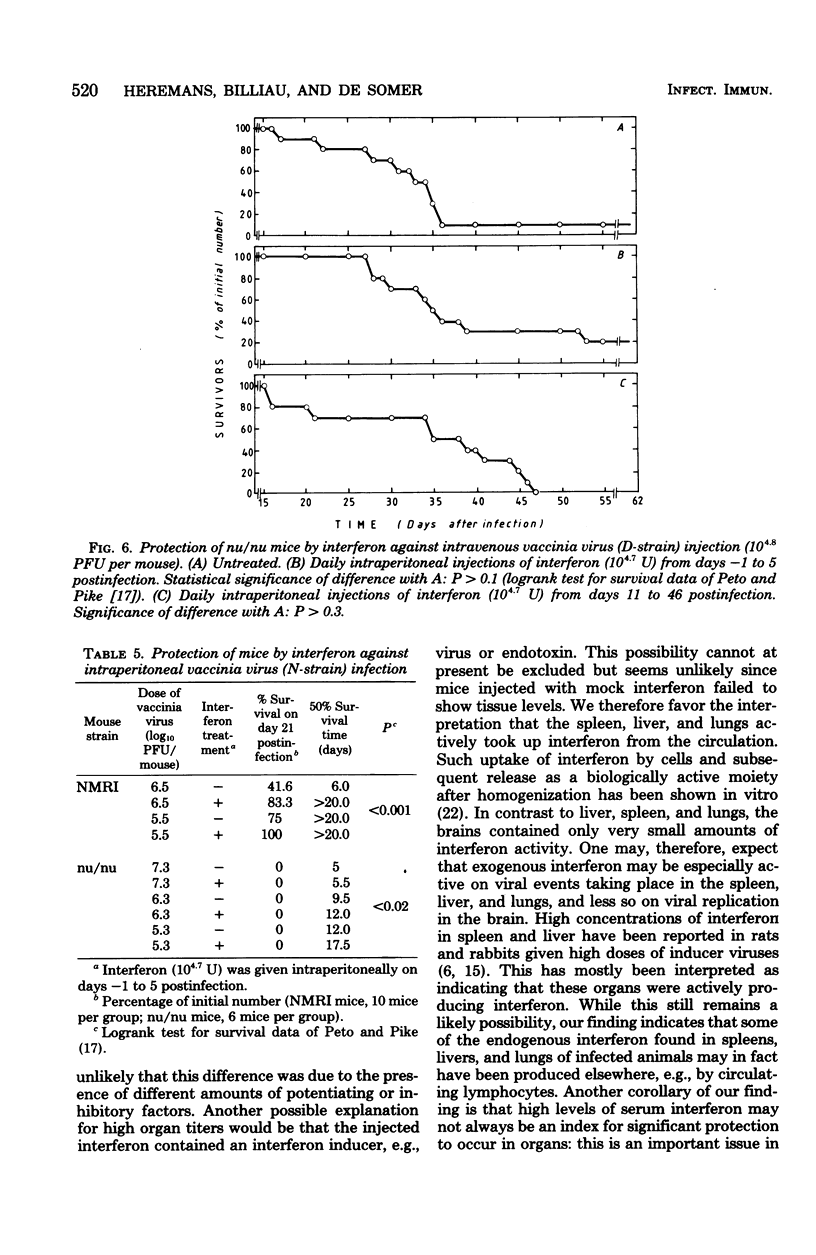
In mice given single intraperitoneal doses of interferon, serum interferon levels peaked at 1 h postinjection and were reduced to zero at about 8 h. The interferon concentrations in spleen, liver, and lungs were about 100-fold higher than could be expected from the amount of serum contained in these organs. In the brain only low levels of antiviral activity were detected. In mice infected intraperitoneally with Mengo virus, viral replication in the brain occurred around day 4 and was accompanied by the appearance of large amounts of interferon (approximately 10(3.25) U/g). This was preceded, however, by viral replication in the spleen and by the appearance of modest amounts of interferon in spleen and serum. In these mice protection could be obtained with relatively small doses of interferon, provided protection could be obtained with relatively small doses of interferon, provided they were given before the time of maximal levels of endogenous serum interferon. In mice infected intranasally with vesicular stomatitis virus, virus replication in the brain started within 24 to 48 h and increased with time; also, small amounts of interferon (10(2) to 10(2.5) U/g) were already detectable on days 1 and 2. The major peak of virus replication in the brain occurred on days 5 to 6 and was accompanied by the appearance of large amounts of interferon (approximately 10(3.25) U/g). In this model early treatment with interferon also provided protection, but only if given in larger doses than in the Mengo virus system. Athymic (nu/nu) mice developed a chronic systemic infection when inoculated with a demotropic strain of vaccinia virus. No interferon was detected in sera, livers, spleens, or lungs of these animals; some mice had low levels of interferon-like antiviral activity in the brain, but no attempt was made to characterize this material. Daily administration of large doses of interferon failed to exert an effect on the development of this chronic disease. Yet, normal (NMRI) mice were protected against acute infection with dermotropic or neurotropic strains of vaccinia virus, and athymic mice were partially protected against acute lethal infection with neurotropic vaccinia virus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billiau A., De Somers P., Edy V. G., De Clercq E., Heremans H. Human fibroblast interferon for clinical trials: pharmacokinetics and tolerability in experimental animals and humans. Antimicrob Agents Chemother. 1979 Jul;16(1):56–63. doi: 10.1128/aac.16.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., De Somer P. Comparative study of the efficacy of different forms of interferon therapy in the treatment of mice challenged intranassaly with vesicular stomatitis virus (VSV). Proc Soc Exp Biol Med. 1971 Oct;138(1):301–307. doi: 10.3181/00379727-138-35884. [DOI] [PubMed] [Google Scholar]
- De Clercq E., De Somer P. Effect of interferon, polyacrylin acid, and polymethacrylic acid on tail lesions on mice infected with vaccinia virus. Appl Microbiol. 1968 Sep;16(9):1314–1319. doi: 10.1128/am.16.9.1314-1319.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., De Somer P. Protective effect of interferon and polyacrylic acid in newborn mice infected with a lethal dose of vesicular stomatitis virus. Life Sci. 1968 Sep 1;7(17):925–933. doi: 10.1016/0024-3205(68)90098-2. [DOI] [PubMed] [Google Scholar]
- De Somer P., Billiau A. Interferon production by the spleen of rats after intravenous injection of Sindbis virus or heat-killed Escherichia coli. Arch Gesamte Virusforsch. 1966;19(2):143–154. doi: 10.1007/BF01241494. [DOI] [PubMed] [Google Scholar]
- Finter N. B. Interferon in mice: protection against small doses of virus. J Gen Virol. 1967 Jul;1(3):395–397. doi: 10.1099/0022-1317-1-3-395. [DOI] [PubMed] [Google Scholar]
- Giovanella B. C., Stehlin J. S. Heterotransplantation of human malignant tumors in "nude" thymusless mice. I. Breeding and maintenance of "nude" mice. J Natl Cancer Inst. 1973 Aug;51(2):615–619. [PubMed] [Google Scholar]
- Gresser I., Fontaine-Brouty-Boyé D., Bourali C., Thomas M. T. A comparison of the efficacy of endogenous, exogenous, and combined endogenous-exogenous interferon in the treatment of mice infected with encephalomyocarditis virus. Proc Soc Exp Biol Med. 1969 Jan;130(1):236–242. doi: 10.3181/00379727-130-33529. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Bandu M. E., Maury C., Brouty-Boyé D. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. I. Rapid evolution of encephalomyocarditis virus infection. J Exp Med. 1976 Nov 2;144(5):1305–1315. doi: 10.1084/jem.144.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Bourali-Maury C. Efficacy of exogenous interferon treatment initiated after onset of multiplication of vesicular stomatitis virus in the brains of mice. J Gen Virol. 1975 Jun;27(3):395–398. doi: 10.1099/0022-1317-27-3-395. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Maury C., Bandu M. T. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. II. Studies with herpes simplex, Moloney sarcoma, vesicular stomatitis, Newcastle disease, and influenza viruses. J Exp Med. 1976 Nov 2;144(5):1316–1323. doi: 10.1084/jem.144.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heremans H., Billiau A., Colombatti A., Hilgers J., de Somer P. Interferon treatment of NZB mice: accelerated progression of autoimmune disease. Infect Immun. 1978 Sep;21(3):925–930. doi: 10.1128/iai.21.3.925-930.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONO Y., HO M. THE ROLE OF THE RETICULOENDOTHELIAL SYSTEM IN INTERFERON FORMATION IN THE RABBIT. Virology. 1965 Jan;25:163–166. doi: 10.1016/0042-6822(65)90268-0. [DOI] [PubMed] [Google Scholar]
- Olsen G. A., Kern E. R., Glasgow L. A., Overall J. C. Effect of treatment with exogenous interferon, polyinosinic acid-polyctyidylic acid or polyinosinic acid-polycytidylic acid-poly-L-lysine complex on encephalomyocarditis virus infections in mice. Antimicrob Agents Chemother. 1976 Oct;10(4):668–676. doi: 10.1128/aac.10.4.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R., Pike M. C. Conservatism of the approximation sigma (O-E)2-E in the logrank test for survival data or tumor incidence data. Biometrics. 1973 Sep;29(3):579–584. [PubMed] [Google Scholar]
- Schellekens H., Weimar W., Cantell K., Stitz L. Antiviral effect of interferon in vivo may be mediated by the host. Nature. 1979 Apr 19;278(5706):742–742. doi: 10.1038/278742a0. [DOI] [PubMed] [Google Scholar]
- Stebbing N. Protection of mice against infection with wild-type Mengo virus and an interferon sensitive mutant (IS-1) by polynucleotides and interferons. J Gen Virol. 1979 Jul;44(1):255–260. doi: 10.1099/0022-1317-44-1-255. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, De Clercq E., De Somer P. Recovery of cell-bound interferon. J Virol. 1972 Oct;10(4):707–712. doi: 10.1128/jvi.10.4.707-712.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Clercq E. Increased resistance of trisomic-21 cells to virus replication: role of interferon. Virology. 1978 May 1;86(1):276–280. doi: 10.1016/0042-6822(78)90028-4. [DOI] [PubMed] [Google Scholar]


