Abstract
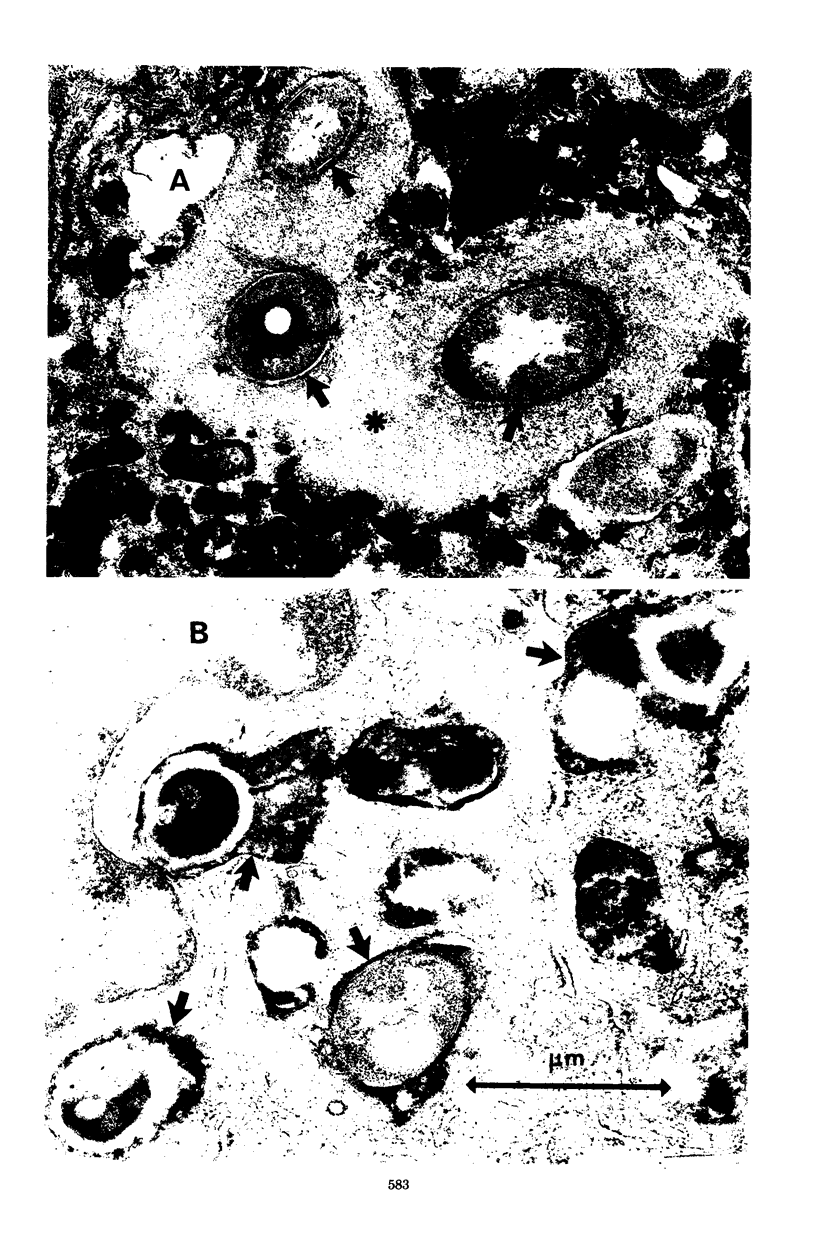
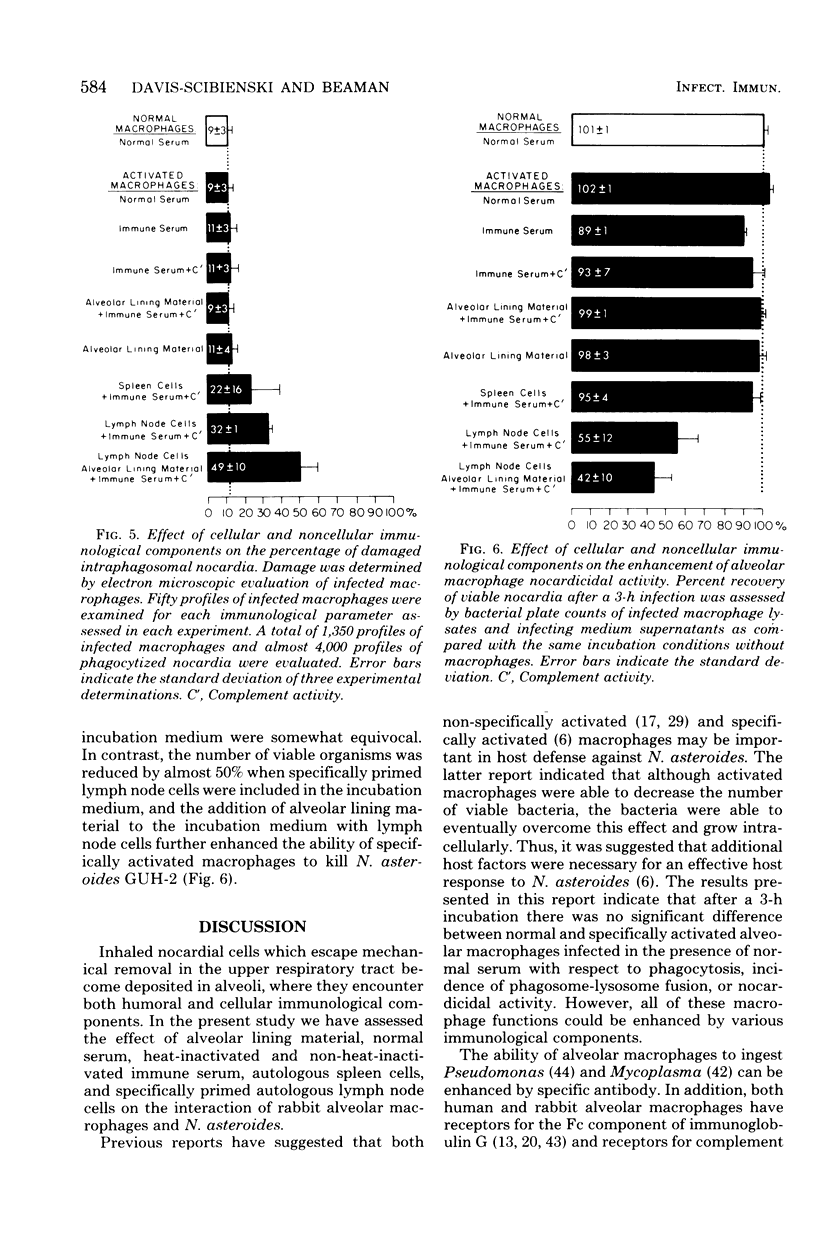
Normal and specifically activated rabbit alveolar macrophages were infected in vitro with Nocardia asteroides GUH-2. In the presence of serum from normal rabbits, no significant differences were noted between normal and activated alveolar macrophages with respect to phagocytosis, incidence of phagosomelysosome fusion, or nocardicidal activity. However, all of these macrophage functions were enhanced by various immunological components. Serum from immunized rabbits enhanced phagocytosis of nocardial cells by activated macrophages, and there was an additional increase in phagocytosis observed when alveolar lining material was present. Complement had no effect on the ability of the macrophages to phagocytize nocardial cells. The greatest percentage of organisms phagocytized was observed when specifically primed lymph node cells, alveolar lining material, and serum from immunized rabbits were present in the incubation medium. N. asteroides GUH-2 inhibited phagosome-lysosome fusion in normal macrophages in the presence of serum from normal rabbits. However, addition of serum from immunized rabbits or the addition of specifically primed lymphocytes increased the amount of phagosome-lysosome fusion, whereas complement had no effect on this fusion process. Nocardial viability was not reduced when either normal or activated macrophages were infected with bacteria in the presence of normal serum, immune serum, or alveolar lining material. However, specifically activated macrophages incubated with primed lymph node cells obtained from immunized rabbits were able to both decrease the number of viable organisms recovered and to increase the incidence and extent of bacterial cell damage. The greatest number of organisms were killed by specifically activated macrophages when the bacterial cells were incubated with primed lymph node cells suspended in immune serum and alveolar lining material. These results indicate that activated macrophages alone are not sufficient to kill ingested N. asteroides GUH-2 and that specifically primed lymphocytes are important in host resistance to nocardial infections.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975 Jul 1;142(1):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbotin J. N., Thomas D. Electron microscopic and kinetic studies dealing with an artificial enzyme membrane. Application to a cytochemical model with the horseradish peroxidase-3,3'-diaminobenzidine system. J Histochem Cytochem. 1974 Nov;22(11):1048–1059. doi: 10.1177/22.11.1048. [DOI] [PubMed] [Google Scholar]
- Beaman B. L., Burnside J., Edwards B., Causey W. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976 Sep;134(3):286–289. doi: 10.1093/infdis/134.3.286. [DOI] [PubMed] [Google Scholar]
- Beaman B. L., Gershwin M. E., Maslan S. Infectious agents in immunodeficient murine models: pathogenicity of Nocardia asteroides in congenitally athymic (nude) and hereditarily asplenic (Dh/+) mice. Infect Immun. 1978 May;20(2):381–387. doi: 10.1128/iai.20.2.381-387.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L. In vitro response of rabbit alveolar macrophages to infection with Nocardia asteroides. Infect Immun. 1977 Mar;15(3):925–937. doi: 10.1128/iai.15.3.925-937.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L. Interaction of Nocardia asteroides at different phases of growth with in vitro-maintained macrophages obtained from the lungs of normal and immunized rabbits. Infect Immun. 1979 Oct;26(1):355–361. doi: 10.1128/iai.26.1.355-361.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Maslan S. Effect of cyclophosphamide on experimental Nocardia asteroides infection in mice. Infect Immun. 1977 Jun;16(3):995–1004. doi: 10.1128/iai.16.3.995-1004.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Smathers M. Interaction of Nocardia asteroides with cultured rabbit alveolar macrophages. Infect Immun. 1976 Apr;13(4):1126–1131. doi: 10.1128/iai.13.4.1126-1131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantey J. R., Hand W. L. Cell-mediated immunity after bacterial infection of the lower respiratory tract. J Clin Invest. 1974 Nov;54(5):1125–1134. doi: 10.1172/JCI107856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen J. E. Migration inhibitory effect of cell-free supernatants from tuberculin-stimulated cultures of human mononuclear leukocytes demonstrated by two-step MIF agarose assay. J Immunol. 1973 Feb;110(2):546–551. [PubMed] [Google Scholar]
- Daughaday C. C., Douglas S. D. Membrane receptors on rabbit and human pulmonary alveolar macrophages. J Reticuloendothel Soc. 1976 Jan;19(1):37–45. [PubMed] [Google Scholar]
- Davis-Scibienski C., Beaman B. L. Interaction of Nocardia asteroides with rabbit alveolar macrophages: association of virulence, viability, ultrastructural damage, and phagosome-lysosome fusion. Infect Immun. 1980 May;28(2):610–619. doi: 10.1128/iai.28.2.610-619.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Scibienski C., Beaman B. L. Interaction of Nocardia asteroides with rabbit alveolar macrophages: effect of growth phase and viability on phagosome-lysosome fusion. Infect Immun. 1980 Jul;29(1):24–29. doi: 10.1128/iai.29.1.24-29.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald G. W., Clyde W. A., Jr, Bienenstock J. Immunoglobulin-containing cells in lungs of hamsters infected with Mycoplasma pneumoniae. J Immunol. 1972 May;108(5):1400–1408. [PubMed] [Google Scholar]
- Filice G. A., Beaman B. L., Remington J. S. Effects of activated macrophages on Nacardia asteroides. Infect Immun. 1980 Feb;27(2):643–649. doi: 10.1128/iai.27.2.643-649.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folb P. I., Timme A., Horowitz A. Nocardia infections in congenitally athymic (nude) mice and in other inbred mouse strains. Infect Immun. 1977 Nov;18(2):459–466. doi: 10.1128/iai.18.2.459-466.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde D. W., Finley T. N., Cline M. J. The pulmonary macrophage in acute leukemia. N Engl J Med. 1974 Apr 18;290(16):875–878. doi: 10.1056/NEJM197404182901603. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Combination of Gomori and Ziehl-Neelsen stains for showing acid phosphatase activity and Mycobacterium tuberculosis in macrophages. Stain Technol. 1969 Jan;44(1):11–14. doi: 10.3109/10520296909063317. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Interference with normal phagosome-lysosome fusion in macrophages, using ingested yeast cells and suramin. Nature. 1975 Jul 3;256(5512):47–49. doi: 10.1038/256047a0. [DOI] [PubMed] [Google Scholar]
- Henney C. S., Waldman R. H. Cell-mediated immunity shown by lymphocytes from the respiratory tract. Science. 1970 Aug 14;169(3946):696–697. doi: 10.1126/science.169.3946.696. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juers J. A., Rogers R. M., McCurdy J. B., Cook W. W. Enhancement of bactericidal capacity of alveolar macrophages by human alveolar lining material. J Clin Invest. 1976 Aug;58(2):271–275. doi: 10.1172/JCI108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krick J. A., Remington J. S. Resistance to infection with Nocardia asteroides. J Infect Dis. 1975 Jun;131(6):665–672. doi: 10.1093/infdis/131.6.665. [DOI] [PubMed] [Google Scholar]
- Krick J. A., Stinson E. B., Remington J. S. Nocardia infection in heart transplant patients. Ann Intern Med. 1975 Jan;82(1):18–26. doi: 10.7326/0003-4819-82-1-18. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaForce F. M. Effect of alveolar lining material on phagocytic and bactericidal activity of lung macrophages against Staphylococcus aureau. J Lab Clin Med. 1976 Nov;88(5):691–699. [PubMed] [Google Scholar]
- LaForce F. M., Kelly W. J., Huber G. L. Inactivation of staphylococci by alveolar macrophages with preliminary observations on the importance of alveolar lining material. Am Rev Respir Dis. 1973 Oct;108(4):784–790. doi: 10.1164/arrd.1973.108.4.784. [DOI] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Mackaness G. B. The J. Burns Amberson LECTURE The induction and expression of cell-mediated hypersensitivity in the lung. Am Rev Respir Dis. 1971 Dec;104(6):813–828. doi: 10.1164/arrd.1971.104.6.813. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani B., Rabinovitch M., Nussenzweig V. Phagocytosis of immune complexes by macrophages. Different roles of the macrophage receptor sites for complement (C3) and for immunoglobulin (IgG). J Exp Med. 1972 Apr 1;135(4):780–792. doi: 10.1084/jem.135.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarroso A. M., Myrvik Q. N. Effect of BCG vaccination on the IgG and complement receptors on rabbit alveolar macrophages. J Reticuloendothel Soc. 1978 Aug;24(2):93–99. [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. A. Trypanosoma cruzi: in vitro induction of macrophage microbicidal activity. J Exp Med. 1978 Jul 1;148(1):288–300. doi: 10.1084/jem.148.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D. L., Harvey R. L., Wheeler J. K. Diagnostic and therapeutic considerations in Nocardia asteroides infection. Medicine (Baltimore) 1974 Sep;53(5):391–401. doi: 10.1097/00005792-197409000-00005. [DOI] [PubMed] [Google Scholar]
- Powell D. A., Muse K. A. Scanning electron microscopy of guinea pig alveolar macrophages: in vitro phagocytosis of Mycoplasma pneumoniae. Lab Invest. 1977 Dec;37(6):535–543. [PubMed] [Google Scholar]
- Reynolds H. Y., Atkinson J. P., Newball H. H., Frank M. M. Receptors for immunoglobulin and complement on human alveolar macrophages. J Immunol. 1975 Jun;114(6):1813–1819. [PubMed] [Google Scholar]
- Reynolds H. Y., Kazmierowski J. A., Newball H. H. Specificity of opsonic antibodies to enhance phagocytosis of Pseudomonas aeruginosa by human alveolar macrophages. J Clin Invest. 1975 Aug;56(2):376–385. doi: 10.1172/JCI108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E. Multiplication of tubercle bacilli within mononuclear phagocytes in tissue cultures derived from normal animals and animals vaccinated with BCG. J Exp Med. 1953 Feb 1;97(2):235–245. doi: 10.1084/jem.97.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin S. B., Stossel T. P. Complement (C3)-activated phagocytosis by lung macrophages. J Immunol. 1978 Apr;120(4):1305–1312. [PubMed] [Google Scholar]
- Skornik W. A., Dressler D. P. Alveolar macrophage function in immunized, burned rats. Arch Surg. 1974 May;108(5):715–719. doi: 10.1001/archsurg.1974.01350290077013. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Brodie S. E., Cohn Z. A. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976 Mar;68(3):665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasi T. B., Jr Secretory immunoglobulins. N Engl J Med. 1972 Sep 7;287(10):500–506. doi: 10.1056/NEJM197209072871008. [DOI] [PubMed] [Google Scholar]
- Tomasi T. B., Jr, Bienenstock J. Secretory immunoglobulins. Adv Immunol. 1968;9:1–96. doi: 10.1016/s0065-2776(08)60441-1. [DOI] [PubMed] [Google Scholar]
- Truitt G. L., Mackaness G. B. Cell-mediated resistance to aerogenic infection of the lung. Am Rev Respir Dis. 1971 Dec;104(6):829–843. doi: 10.1164/arrd.1971.104.6.829. [DOI] [PubMed] [Google Scholar]
- Weidner E. Interactions between Encephalitozoon cuniculi and macrophages. Parasitophorous vacuole growth and the absence of lysosomal fusion. Z Parasitenkd. 1975 Aug 21;47(1):1–9. doi: 10.1007/BF00418060. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Anacker R. L., Ribi E. Macrophage Migration Inhibition Studies with Cells from Mice Vaccinated with Cell Walls of Mycobacterium bovis BCG: Relationship Between Inhibitory Activity of Lung Cells and Resistance to Airborne Challenge with Mycobacterium tuberculosis H37Rv. Infect Immun. 1970 Jun;1(6):595–599. doi: 10.1128/iai.1.6.595-599.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]