Abstract
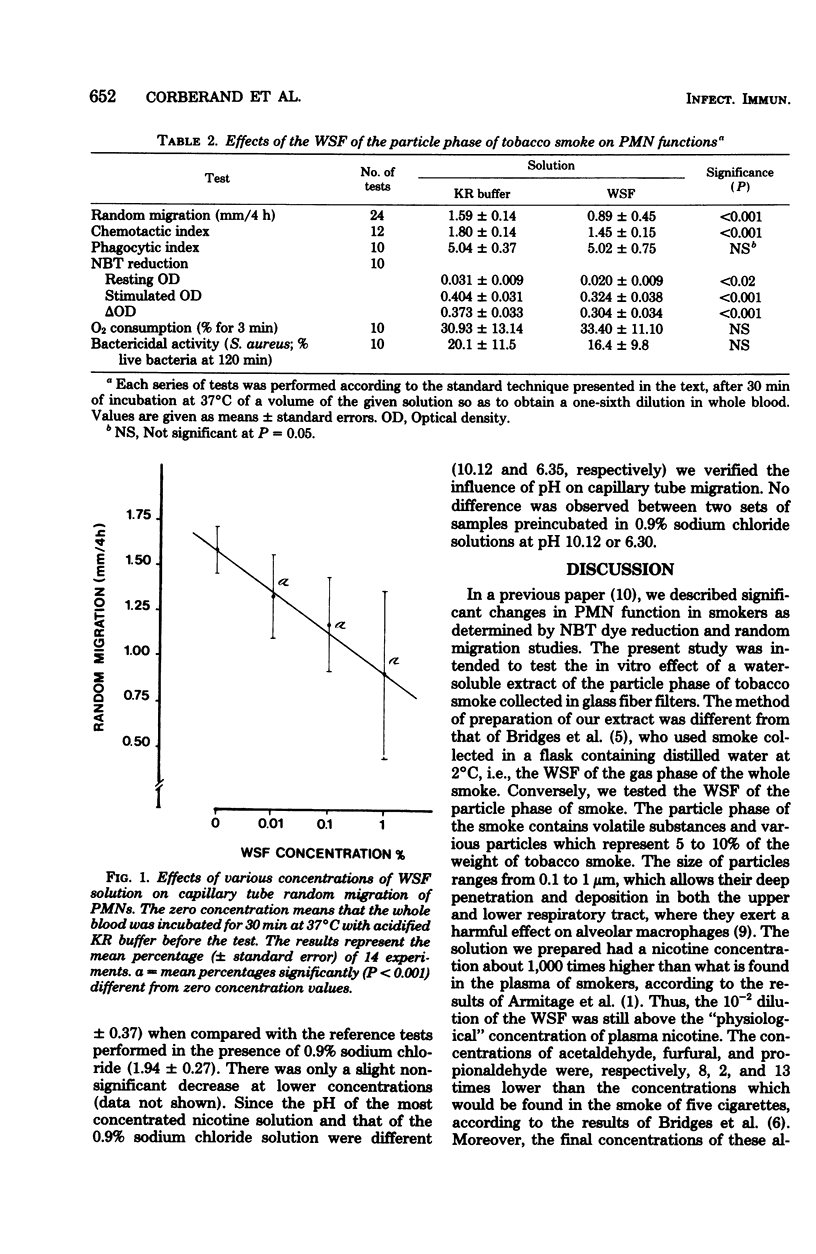
The function of polymorphonuclear leukocytes (PMNs) has previously been shown to be impaired in smokers in comparison with healthy nonsmokers. Potent inhibition of PMN chemotaxis has been achieved with whole tobacco smoke, the gas phase of smoke, and a water-soluble extract of whole smoke. In the present work several aspects of PMN function were studied after exposure to water-soluble fraction of the particle phase of tobacco smoke collected on glass fiber filters. These tests included capillary tube random migration, chemotaxis under agarose, phagocytosis of yeasts, Nitro Blue Tetrazolium dye reduction, and whole-blood bactericidal activity. The water extract of the particle fraction of smoke had a high content of nicotine when compared with the levels achieved in plasma of smokers and a much lower concentration of aldehydes when compared with the gas phase of smoke. It had no cytotoxic effect and did not affect phagocytosis, oxygen consumption, or bactericidal activity. Nitro Blue Tetrazolium reduction of both resting and stimulated PMNs was significantly decreased only with the most concentrated solution. The tested solutions exerted a dose-related depressive effect on capillary tube random migration, whereas the random migration measured in the agarose chemotaxis test was normal. Nevertheless, the chemotactic response to a caseine solution was significantly decreased. The same tests were performed in the presence of several concentrations of a nicotine solution and the only test to be affected was the capillary tube random migration, and, that only at a very high concentration. The results of this study contribute to the more precise delineation of the extent of the dysfunction of PMNs exposed to tobacco smoke components and indicate that deleterious products are released from the particle phase of the smoke, which deposits all along the respiratory tree.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitage A. K., Dollery C. T., George C. F., Houseman T. H., Lewis P. J., Turner D. M. Absorption and metabolism of nicotine from cigarettes. Br Med J. 1975 Nov 8;4(5992):313–316. doi: 10.1136/bmj.4.5992.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Blue M. L., Janoff A. Possible mechanisms of emphysema in cigarette smokers. Release of elastase from human polymorphonuclear leukocytes by cigarette smoke condensate in vitro. Am Rev Respir Dis. 1978 Feb;117(2):317–325. doi: 10.1164/arrd.1978.117.2.317. [DOI] [PubMed] [Google Scholar]
- Bridges R. B., Kraal J. H., Huang L. J., Chancellor B. M. Effects of tobacco smoke on chemotaxis and glucose metabolism of polymorphonuclear leukocytes. Infect Immun. 1977 Jan;15(1):115–123. doi: 10.1128/iai.15.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges R. B., Kraal J. H., Huang L. J., Chancellor M. B. Effects of cigarette smoke components on in vitro chemotaxis of human polymorphonuclear leukocytes. Infect Immun. 1977 Apr;16(1):240–248. doi: 10.1128/iai.16.1.240-248.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro O., Andriole V. T., Finch S. C. Whole blood phagocytic and bactericidal activity for Staphylococcus aureus. J Lab Clin Med. 1972 Dec;80(6):857–870. [PubMed] [Google Scholar]
- Chrétien J. Le rôle du tabac dans les perturbations des défenses immunitaires pulmonaires. Nouv Presse Med. 1979 Jun 7;8(25):2131–2134. [PubMed] [Google Scholar]
- Corberand J., Nguyen F., Do A. H., Dutau G., Laharrague P., Fontanilles A. M., Gleizes B. Effect of tobacco smoking on the functions of polymorphonuclear leukocytes. Infect Immun. 1979 Mar;23(3):577–581. doi: 10.1128/iai.23.3.577-581.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichel B., Shahrik H. A. Tobacco smoke toxicity: loss of human oral leukocyte function and fluid-cell metabolism. Science. 1969 Dec 12;166(3911):1424–1428. doi: 10.1126/science.166.3911.1424. [DOI] [PubMed] [Google Scholar]
- Fehr J., Dahinden C. Modulating influence of chemotactic factor-induced cell adhesiveness on granulocyte function. J Clin Invest. 1979 Jul;64(1):8–16. doi: 10.1172/JCI109466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KETCHEL M. M., FAVOUR C. B. The acceleration and inhibition of migration of human leucocytes in vitro by plasma protein fractions. J Exp Med. 1955 Jun 1;101(6):647–663. doi: 10.1084/jem.101.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Noble R. C., Penny B. B. Comparison of leukocyte count and function in smoking and nonsmoking young men. Infect Immun. 1975 Sep;12(3):550–555. doi: 10.1128/iai.12.3.550-555.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R. J., White R. R., Senior R. M., Levine E. A. Elastase release from human alveolar macrophages: comparison between smokers and nonsmokers. Science. 1977 Oct 21;198(4314):313–314. doi: 10.1126/science.910131. [DOI] [PubMed] [Google Scholar]
- Shea J. W., Huber G. L., Holmes L., Homans A. The effect of experimental tobacco smoke inhalation on in vitro alveolar macrophage bactericidal function. J Lab Clin Med. 1978 Aug;92(2):270–282. [PubMed] [Google Scholar]
- Spilberg I., Mandell B., Hoffstein S. A proposed model for chemotactic deactivation: evidence for microtubule modulation of polymorphonuclear leukocyte chemotaxis. J Lab Clin Med. 1979 Aug;94(2):361–369. [PubMed] [Google Scholar]


