Abstract
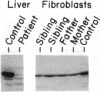
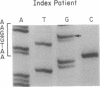
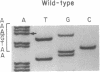
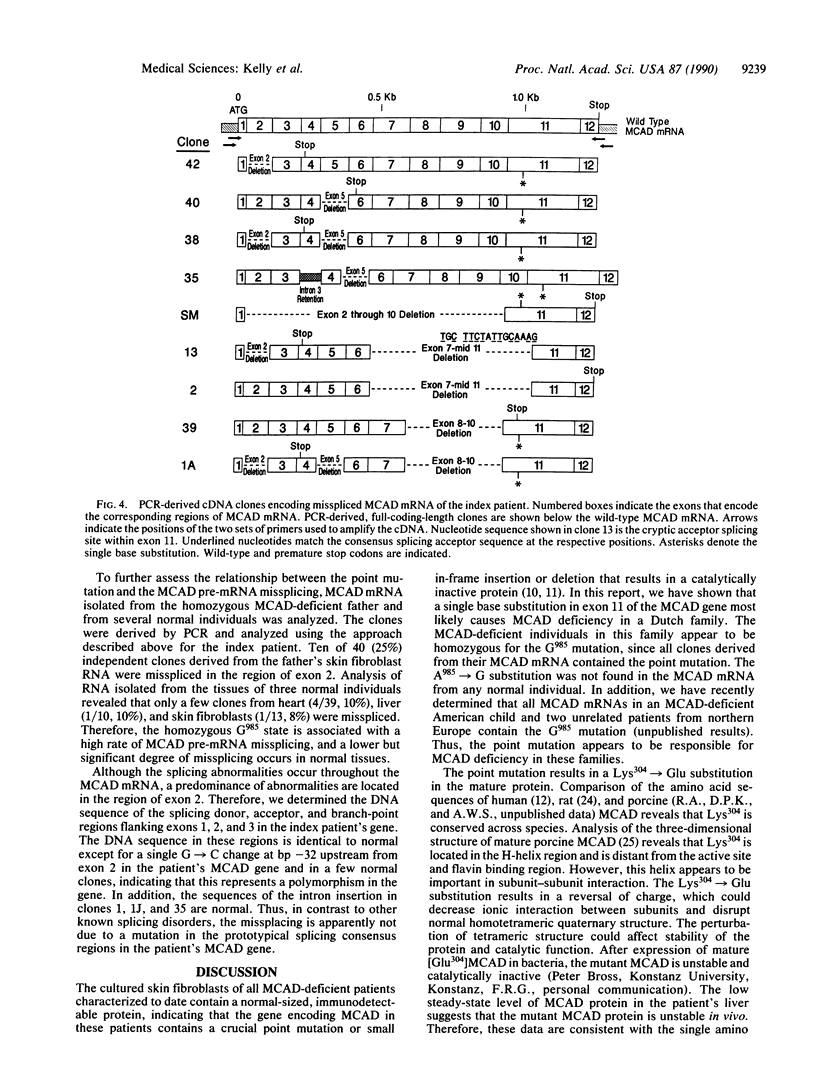
Deficiency of medium-chain acyl-CoA dehydrogenase (MCAD) is a common inherited defect in energy metabolism. Characterization of the mRNA encoding MCAD in a Dutch MCAD-deficient patient revealed an A----G change at nucleotide position 985 of the MCAD mRNA coding region. This point mutation results in the substitution of a glutamic acid for a lysine at amino acid position 304 of the mature protein. The single base change was not found in any wild-type MCAD mRNAs. A mutant allele-specific oligonucleotide probe was used in a hybridization analysis of amplified genomic DNA of MCAD-deficient family members, a carrier, and normal individuals. The hybridization analysis specifically identified individuals who were heterozygotes or homozygotes. In addition to the point mutation, a significant proportion of the index patient's MCAD mRNA contained a variety of deletions and insertions as a result of exon skipping and intron retention. The missplicing occurred in multiple regions throughout the MCAD mRNA. Analysis of the patient's MCAD gene in the regions where the missplicing occurred most frequently did not reveal a mutation in the splicing acceptor or donor sites. Therefore, the molecular characterization of this family revealed a crucial point mutation in the MCAD gene and an unusual abnormality in MCAD pre-mRNA splicing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bougnères P. F., Rocchiccioli F., Kølvraa S., Hadchouel M., Lalau-Keraly J., Chaussain J. L., Wadman S. K., Gregersen N. Medium-chain acyl-CoA dehydrogenase deficiency in two siblings with a Reye-like syndrome. J Pediatr. 1985 Jun;106(6):918–921. doi: 10.1016/s0022-3476(85)80237-7. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Busslinger M., Moschonas N., Flavell R. A. Beta + thalassemia: aberrant splicing results from a single point mutation in an intron. Cell. 1981 Dec;27(2 Pt 1):289–298. doi: 10.1016/0092-8674(81)90412-8. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Duran M., Hofkamp M., Rhead W. J., Saudubray J. M., Wadman S. K. Sudden child death and 'healthy' affected family members with medium-chain acyl-coenzyme A dehydrogenase deficiency. Pediatrics. 1986 Dec;78(6):1052–1057. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Howat A. J., Bennett M. J., Variend S., Shaw L., Engel P. C. Defects of metabolism of fatty acids in the sudden infant death syndrome. Br Med J (Clin Res Ed) 1985 Jun 15;290(6484):1771–1773. doi: 10.1136/bmj.290.6484.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Hale D. E., Keese S. M., Coates P. M., Tanaka K. Biosynthesis of variant medium chain acyl-CoA dehydrogenase in cultured fibroblasts from patients with medium chain acyl-CoA dehydrogenase deficiency. Pediatr Res. 1986 Sep;20(9):843–847. doi: 10.1203/00006450-198609000-00007. [DOI] [PubMed] [Google Scholar]
- Kelly D. P., Kim J. J., Billadello J. J., Hainline B. E., Chu T. W., Strauss A. W. Nucleotide sequence of medium-chain acyl-CoA dehydrogenase mRNA and its expression in enzyme-deficient human tissue. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4068–4072. doi: 10.1073/pnas.84.12.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. J., Wu J. Structure of the medium-chain acyl-CoA dehydrogenase from pig liver mitochondria at 3-A resolution. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6677–6681. doi: 10.1073/pnas.85.18.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kølvraa S., Gregersen N., Christensen E., Hobolth N. In vitro fibroblast studies in a patient with C6-C10-dicarboxylic aciduria: evidence for a defect in general acyl-CoA dehydrogenase. Clin Chim Acta. 1982 Nov 24;126(1):53–67. doi: 10.1016/0009-8981(82)90361-8. [DOI] [PubMed] [Google Scholar]
- Matsubara Y., Kraus J. P., Ozasa H., Glassberg R., Finocchiaro G., Ikeda Y., Mole J., Rosenberg L. E., Tanaka K. Molecular cloning and nucleotide sequence of cDNA encoding the entire precursor of rat liver medium chain acyl coenzyme A dehydrogenase. J Biol Chem. 1987 Jul 25;262(21):10104–10108. [PubMed] [Google Scholar]
- Matsubara Y., Kraus J. P., Yang-Feng T. L., Francke U., Rosenberg L. E., Tanaka K. Molecular cloning of cDNAs encoding rat and human medium-chain acyl-CoA dehydrogenase and assignment of the gene to human chromosome 1. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6543–6547. doi: 10.1073/pnas.83.17.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Rhead W. J., Amendt B. A., Fritchman K. S., Felts S. J. Dicarboxylic aciduria: deficient [1-14C]octanoate oxidation and medium-chain acyl-CoA dehydrogenase in fibroblasts. Science. 1983 Jul 1;221(4605):73–75. doi: 10.1126/science.6857268. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley C. A., Hale D. E., Coates P. M., Hall C. L., Corkey B. E., Yang W., Kelley R. I., Gonzales E. L., Williamson J. R., Baker L. Medium-chain acyl-CoA dehydrogenase deficiency in children with non-ketotic hypoglycemia and low carnitine levels. Pediatr Res. 1983 Nov;17(11):877–884. doi: 10.1203/00006450-198311000-00008. [DOI] [PubMed] [Google Scholar]
- Strauss A. W., Duran M., Zhang Z. F., Alpers R., Kelly D. P. Molecular analysis of medium chain acyl-CoA dehydrogenase deficiency. Prog Clin Biol Res. 1990;321:609–623. [PubMed] [Google Scholar]
- Treem W. R., Witzleben C. A., Piccoli D. A., Stanley C. A., Hale D. E., Coates P. M., Watkins J. B. Medium-chain and long-chain acyl CoA dehydrogenase deficiency: clinical, pathologic and ultrastructural differentiation from Reye's syndrome. Hepatology. 1986 Nov-Dec;6(6):1270–1278. doi: 10.1002/hep.1840060608. [DOI] [PubMed] [Google Scholar]
- Treisman R., Orkin S. H., Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983 Apr 14;302(5909):591–596. doi: 10.1038/302591a0. [DOI] [PubMed] [Google Scholar]
- Treisman R., Proudfoot N. J., Shander M., Maniatis T. A single-base change at a splice site in a beta 0-thalassemic gene causes abnormal RNA splicing. Cell. 1982 Jul;29(3):903–911. doi: 10.1016/0092-8674(82)90452-4. [DOI] [PubMed] [Google Scholar]