Abstract
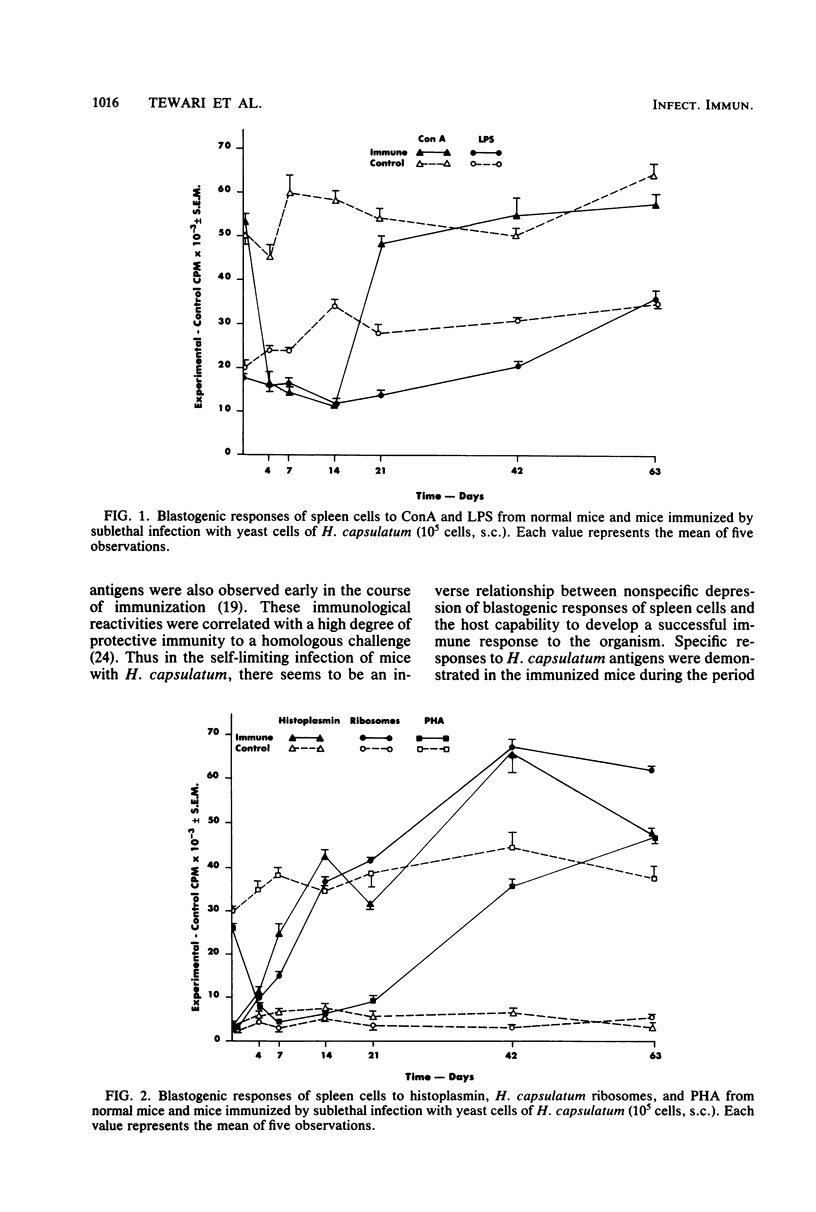
Blastogenic responses of spleen cells to histoplasmin and ribosomal antigens and to the mitogens concanavalin A. phytohemagglutinin, and lipopolysaccharide were studied in normal and immunized mice (10(5) live yeast cells of Histoplasma capsulatum given by the subcutaneous route). Cells (10(6) per well) were cultured with and without antigens and mitogens in microtiter plates with RPMI 1640-5% heat-inactivated normal mouse serum for 72 h at 37 degrees C. Cells were harvested after a 16- to 18-h pulse with 1 microCi of [3H]thymidine (6.7 Ci/mol), and thymidine incorporation was measured by scintillation counting. The initial blastogenic response to concanavalin A (54 X 10(3) cpm) was suppressed (P less than 0.001) from 4 to 14 days post-immunization and returned to control levels on day 21. The response to phytohemagglutinin was suppressed up to 21 days. Lipopolysaccharide responses, however, were affected to a lesser degree. Blastogenic responses to histoplasmin and H. capsulatum ribosomes were similar on day 0 in normal and immune lymphocytes, but by day 4 cells from immunized mice were more responsive (P less than 0.01). The maximum response to H. capsulatum antigens was detected on day 42 and was 9- to 16-fold higher than in controls. These results demonstrate in vitro responses of primed lymphocytes on exposure to H. capsulatum antigens and suppressed responses to mitogens during early stages of the immune response.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford R. H., Goodwin R. A. Patterns of immune response in chronic pulmonary histoplasmosis. J Infect Dis. 1972 Mar;125(3):269–275. doi: 10.1093/infdis/125.3.269. [DOI] [PubMed] [Google Scholar]
- Artz R. P., Bullock W. E. Immunoregulatory responses in experimental disseminated histoplasmosis: depression of T-cell-dependent and T-effectory responses by activation of splenic suppressor cells. Infect Immun. 1979 Mar;23(3):893–902. doi: 10.1128/iai.23.3.893-902.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artz R. P., Bullock W. E. Immunoregulatory responses in experimental disseminated histoplasmosis: lymphoid organ histopathology and serological studies. Infect Immun. 1979 Mar;23(3):884–892. doi: 10.1128/iai.23.3.884-892.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A. Cross-reactivity between antigens of Coccidioides immitis, Histoplasma capsulatum and Blastomyces dermatitidis in lymphocyte transformation assays. Infect Immun. 1979 Sep;25(3):932–938. doi: 10.1128/iai.25.3.932-938.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A. Immunologic studies of patients with histoplasmosis. Am Rev Respir Dis. 1979 Jul;120(1):143–149. doi: 10.1164/arrd.1979.120.1.143. [DOI] [PubMed] [Google Scholar]
- Domer J. E. In vivo and in virto cellular responses to cytoplasmic and cell wall antigens of Histoplasma capsulatum in artificially immunized or infected guinea pigs. Infect Immun. 1976 Mar;13(3):790–799. doi: 10.1128/iai.13.3.790-799.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eardley D. D., Jayawardena A. N. Suppressor cells in mice infected with Trypanosoma brucei. J Immunol. 1977 Sep;119(3):1029–1033. [PubMed] [Google Scholar]
- Ehrhard H. B., Pine L. Factors influencing the production of H and M antigens by Histoplasma capsulatum: development and evaluation of a shake culture. Appl Microbiol. 1972 Feb;23(2):236–249. doi: 10.1128/am.23.2.236-249.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhard H. B., Pine L. Factors influencing the production of H and M antigens by Histoplasma capsulatum: effect of physical factors and composition of medium. Appl Microbiol. 1972 Feb;23(2):250–261. doi: 10.1128/am.23.2.250-261.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feit C., Tewari R. P. Immunogenicity of Ribosomal Preparations from Yeast Cells of Histoplasma capsulatum. Infect Immun. 1974 Nov;10(5):1091–1097. doi: 10.1128/iai.10.5.1091-1097.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson K. M., Terry R. J. Immunodepression and the course of infection of a chronic Trypanosoma brucei infection in mice. Parasite Immunol. 1979 Winter;1(4):317–326. doi: 10.1111/j.1365-3024.1979.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H. Suppressor cells in experimentally trypanosomiasis. Nature. 1977 Feb 10;265(5594):539–541. doi: 10.1038/265539a0. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Herberman R. B., Glaser M., Lavrin D. H. Suppression of in vitro lymphocyte stimulation in mice bearing primary Moloney sarcoma virus-induced tumors. Cell Immunol. 1974 Jul;13(1):32–40. doi: 10.1016/0008-8749(74)90224-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nickerson D. A., Havens R. A., Bullock W. E. Immunoregulation in disseminated histoplasmosis: characterization of splenic suppressor cell populations. Cell Immunol. 1981 May 15;60(2):287–297. doi: 10.1016/0008-8749(81)90270-7. [DOI] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez H., Pocino M., Malavé I. Nonspecific immunodepression and protective immunity in mice infected with Leishmania mexicana. Infect Immun. 1981 May;32(2):415–419. doi: 10.1128/iai.32.2.415-419.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Paul S., Van Scoy R. E., Hermans P. E. Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest. 1976 Feb;57(2):319–328. doi: 10.1172/JCI108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. I. Responsiveness to and surface binding of concanavalin A and phytohemagglutinin. J Immunol. 1972 Jan;108(1):1–17. [PubMed] [Google Scholar]
- Strickland G. T., Ahmed A., Sell K. W. Blastogenic response of Toxoplasma-infected mouse spleen cells to T- and B-cell mitogens. Clin Exp Immunol. 1975 Oct;22(1):167–176. [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Watanabe N., Kobayashi A. Nonspecific suppression of primary antibody responses and presence of plastic-adherent suppressor cells in Toxoplasma gondii-infected mice. Infect Immun. 1981 Oct;34(1):30–35. doi: 10.1128/iai.34.1.30-35.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari R. P., Sharma D. K., Mathur A. Significance of thymus-derived lymphocytes in immunity elicited by immunization with ribosomes or live yeast cells of Histoplasma capsulatum. J Infect Dis. 1978 Nov;138(5):605–613. doi: 10.1093/infdis/138.5.605. [DOI] [PubMed] [Google Scholar]
- Tewari R. P., Sharma D., Solotorovsky M., Lafemina R., Balint J. Adoptive transfer of immunity from mice immunized with ribosomes or live yeast cells of Histoplasma capsulatum. Infect Immun. 1977 Mar;15(3):789–795. doi: 10.1128/iai.15.3.789-795.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R. Evidence for two distinct populations of suppressor cells in the spleens of Mycobacterium bovis BCG-Sensitized mice. Infect Immun. 1981 Nov;34(2):315–322. doi: 10.1128/iai.34.2.315-322.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L. Protein synthesis during fungal spore germination. I. Characteristics of an in vitro phenylalanine incorporating system prepared from germinated spores of Botryodiplodia theobromae. Arch Biochem Biophys. 1968 Apr;125(1):13–21. doi: 10.1016/0003-9861(68)90632-2. [DOI] [PubMed] [Google Scholar]


