Abstract
Aging as a research pursuit is fairly new compared with traditional lines of medical research. A growing field of investigators is focused on understanding how changes in tissue biology, physiology, and systemic homeostasis, conspire to create increased vulnerability to disease as a function of age. Aging research as a discipline is necessarily broad; in part because aging itself is multi-faceted and in part because different model systems are employed to define the underlying biology. In this review we outline aspects of aging research that are likely to uncover the pivotal events leading to age-related disease vulnerability. We focus on studies of human aging and discuss the value of research on caloric restriction, an intervention with proven efficacy in delaying aging. We propose that studies such as these will deliver target factors and processes that create vulnerability in human aging, an advance that would potentially be transformative in clinical care.
Keywords: Caloric restriction, Aging, Metabolism, Humans, Nonhuman primates
Highlights
-
•
Caloric restriction delays aging and is a powerful tool for understanding the biology of age-related disease vulnerability.
-
•
Mechanisms induced by CR hold promise for the development of interventions for age-associated disease.
-
•
Nonhuman primates are an excellent model for transition from biological insight to human clinical application.
1. Introduction
The dramatic increase in average life expectancy has led to a rapid rise in the aging population across the globe (Christensen et al., 2009). Age is a robust and independent risk factor for a range of non-communicable diseases like cancer, diabetes, cardiovascular disease, and neurodegenerative disease, and so it follows that this newfound increase in longevity creates a substantial burden in disease incidence and health care costs (Bloom et al., 2015). Overwhelming evidence suggests that processes intrinsic to aging contribute to the pathogenesis of age-related diseases. Ongoing international efforts have made great strides in advancing our knowledge of the biology of aging and several “hallmarks” of aging have been identified that may play a causative role in the age-related increase in disease vulnerability (Kennedy et al., 2014, Lopez-Otin et al., 2013). These age-related changes include fundamental aspects of biology such as metabolic dysfunction, genomic instability, failure of quality control mechanisms, disruption in cellular pathways controlling growth and recycling, failure in integrity of cell-cell communication, and loss of regenerative capacity. Consequent changes in metabolic homeostasis and inflammatory tone are thought to further compound these primary defects of age (Finkel, 2015, Franceschi and Campisi, 2014), negatively influencing the tissue microenvironment to create a permissive state for disease incidence and progression.
These last few years have seen a shift in emphasis from the investigation of individual age-related diseases in isolation toward a broader context to define the basic biology of aging. The concept behind the recently coined pursuit of GeroScience (Burch et al., 2014) is that a strategy to delay the aging process itself would decrease vulnerability across the age-related disease spectrum leading to lower morbidity and comorbidity. Indeed the concept that aging might be a suitable drug target in a clinical context is gaining traction and there is considerable effort being applied to bring this idea to fruition (Longo et al., 2015). One of the most valuable tools in aging research is caloric restriction (CR), a proven intervention to delay aging and age-related disease (Fig. 1). If we could understand what mechanisms are employed by CR to impinge on the aging process we could potentially identify causal networks that contribute to the increase in disease vulnerability as a function of normative aging.
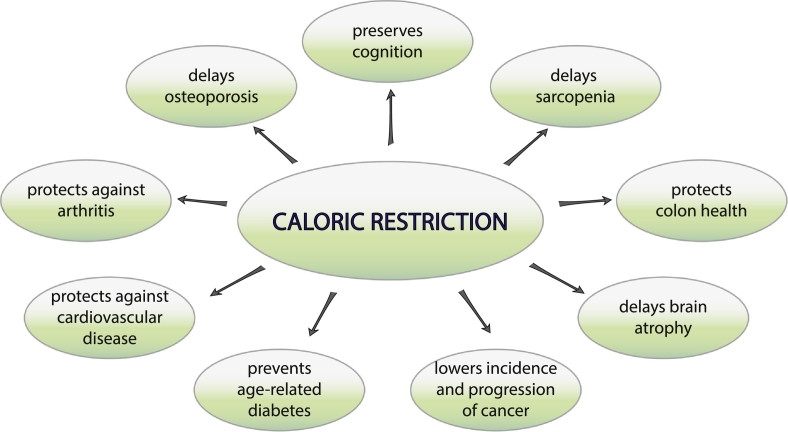
Fig. 1.
Caloric Restriction (CR) delays the onset of age-related diseases. Studies in rodents and nonhuman primates have revealed a beneficial effect of CR on a diverse set of conditions related to human aging.
2. Human Aging Studies
Several large-scale longitudinal aging studies have contributed enormously to our current understanding of the physiological changes during aging and their impact on health in old age. The Baltimore Longitudinal Study of Aging started in 1958, even before the foundation of the National Institute on Aging NIA in 1974. The study set out to determine the trajectory of change as a function of normal aging. Over the years 1300 participants have been monitored longitudinally, with health, cognitive and functional assessments conducted periodically. More recently, auxiliary studies have been conducted to identify the molecular signature of age related functional declines, including the investigation of the ability of biological markers such as serum metabolites to index physical function (Moaddel et al., 2016). Studies such as these will contribute new biomarkers with utility for diagnosis and treatment efficacy monitoring but also have potential to reveal the underlying biology of the age-related disorders. The Health Aging and Body Composition (Health ABC) is a longitudinal study of over 3000 subjects in the 70–79 year old age range that were recruited in 1997 and followed for a decade and a half. Data from this study have featured in hundreds of publications since 1999. Early reports focused on functional decline with age (Simonsick et al., 2001) but as the study progressed reports of interactions among measured parameters and their association with disease have emerged (Beavers et al., 2013). The survey of Midlife Development in the US (MIDUS) was launched in 1995 with the overarching goal to discover the contributions of behavioral, psychological, and social factors to variation in health and well-being as a function of age (Radler, 2014). A repository of data and specimens collected from subjects in the study has enabled the investigation of biological factors to determine how these impact aging and health, such as interactions among biological indices as predictors of health and the role of inflammatory tone (Elliot and Chapman, 2016).
The Wisconsin Longitudinal Study is a long-term study of a random sample of 10,317 men and women who graduated from Wisconsin high schools in 1957 (Herd et al., 2014). Survey data were collected from the original respondents and a selected sibling and from their spouses. Health, social, and economic data have been collected, with more recent initiatives including genetic studies of a sub-group of the cohort. The Health and Retirement Study based at the University of Michigan was initiated in 1992 and focused on the 51–61 year age group. Subjects, now in their 70s and 80s, were interviewed every two years. Additional groups were added later including a cohort older than 70, a cohort of subjects who had birth dates in the years of the depression who were in their 60s at the time of recruitment, and the “war babies” cohort who were in their early 50s. Currently the study has captured data from ~ 20,000 persons. Over the years the investigation has expanded to include on site physical assessments, biomarkers and genetics, allowing the biological underpinnings of age-related disease and disorders to be uncovered (Duchowny et al., 2017, Mezuk et al., 2016). Frameworks such as the studies described above have great potential to uncover factors contributing to multi-morbidity, a key aspect in geriatric care and a major factor in loss of independence (Fabbri et al., 2015). As more molecular level data emerge from analysis of collected biospecimens from each of these and other longitudinal aging studies we can anticipate the emergence of a whole new perspective on aging biology in humans at unprecedented resolution.
The Lothian Birth cohorts of 1921 and 1936 are studies that were initiated in 1999 and 2004 respectively, originally aimed at uncovering the genetic determinants of cognitive aging. These are follow up studies to the Scottish mental survey that tested the intelligence of almost all children aged 11 that attended Scottish school in 1932 and 1947 and were later recruited for the longitudinal study at the mean age of 79 and 70. In addition to genetic determinants, several other factors like physical fitness, inflammatory profile, renal function, VitB12 and folate, psycho-social and economic status were found to be associated with lifetime cognitive aging (Deary et al., 2012). The Leiden 85 + longitudinal study began in 1997 with 599 individuals aged 85 and older inhabiting the city of Leiden in Netherlands. They were followed up for the next five years with particular focus on inflammation and vascular factors linked with aging. These studies uncovered an association between atherosclerosis and dementia in old age (Vinkers et al., 2005). Extending the Leiden 85 + study, the Newcastle 85 + study comprises over 1000 individuals that were recruited in 2006 at the age of 85. This study included interested participants without regard to their health status thereby avoiding selection bias in the cohort. This design captured the broad spectrum of health in the oldest individuals, with reported outcomes linked to various biological and social factors (Collerton et al., 2007).
Another highly informative avenue of investigation includes the study of exceptional longevity. These studies investigate parameters related to aging in centenarians, semi-super agers > 105 years of age, and super agers > 110 years of age. The New England Centenarian Study was founded in 1995 at Boston University and expanded to the multi-center Long Life Family Study established in 2006. The latter is a prospective study of 5000 subjects from > 500 families that are enriched for exceptional longevity. Notably the New England Centenarian study reported that the extension of longevity is associated with a compression in morbidity so that in addition to having a longer life these centenarians have a longer period of health (Andersen et al., 2012), suggesting that multiple aspects of aging are off-set and delayed in this cohort. Genetic analysis identified the TOMM40/ApoE locus as important for very long life (Sebastiani et al., 2012). ApoE is the gene encoding apolipoprotein E involved in lipid transport and cholesterol metabolism, and the importance of the TOMM40/APOE locus in longevity was confirmed in a meta-analysis of data from 5 cententarian studies from USA, Europe and Japan (Sebastiani et al., 2013). The Longevity Genes Project based in the Albert Einstein College of Medicine investigates the basis for longevity in a founder population of Ashkenazi Jews. The cohort includes over 500 individuals with exceptional longevity, over 700 of their offspring, and a further 600 unrelated individuals. Here also a connection to lipid metabolism has been made including genes involved in lipoproteins and cholesterol metabolism (Barzilai et al., 2003), and the adipose derived signaling peptide adiponectin, a regulator of lipid fuel utilization (Atzmon et al., 2008). Interestingly, adiponectin is implicated in the mechanisms of CR and linked to enhanced longevity in rodents (see below). Adiponectin was also identified as a longevity associated gene in a Polish Centenarian study (Roszkowska-Gancarz et al., 2012). The Okinawa Centenarian Study (OCS) investigates the role of genetics and lifestyle factors in regulation of longevity in a distinct Japanese cohort. The OCS was initiated in 1975 and since then over 900 centenarians have been enrolled (Willcox et al., 2016). A candidate approach identified FOXO3a, a transcription factor linked to insulin signaling as being important in longevity (Willcox et al., 2008), a finding that was confirmed in the Southern Italian Centenarian study (Anselmi et al., 2009). Signaling pathways down stream of insulin and insulin like growth factor (IGF-1) have been clearly linked to longevity in short lived species, where genetic studies in a range of species have demonstrated the ability to target this pathway to manipulate aging (Kenyon, 2010).
Genome-wide association studies (GWAS) for longevity and healthy aging have only recently moved to the fore in gerontology research. The first consistent association with longevity emerging from GWAS is APOE (Schachter et al., 1994), a gene that has established links to Alzheimer's disease (Beecham et al., 2009). APOE has subsequently been linked to a variety of conditions and a lipoprotein metabolic theme appears to be shared among the major disorders of aging (Johnson et al., 2015). GWAS has also been applied to identify determinants of circulating lipid levels, the European Network for Genetic and Genomic Epidemiology (ENGAGE) conducted a meta-analysis to identify genes associated with serum lipid burden where many of the candidate genes aligned beautifully with the known biology of lipid metabolic regulation in addition to novel roles for genes previously unidentified as being involved in lipid biology (Aulchenko et al., 2009). Studies investigating genetic determinants of body fat distribution identified numerous new genes of interest, confirming the long suspected links between adipose tissue and metabolic dysfunction (Locke et al., 2015, Shungin et al., 2015). Studies of complex traits have also proved informative, for example the Cohorts for Heath and Aging Research in Genomic Epidemiology (CHARGE) consortium used a combination of GWAS with subsequent quantitative trait locus (QTL) analysis to identify several genes associated with variance in gait speed (Ben-Avraham et al., 2017). Network analysis linked these genes to growth and inflammatory signaling, including factors involves in ion transport, metabolism, and cell structure. A similar study of dynapenia, the loss of muscle strength, identified CEBP (CCAAT/enhancer-binding protein beta) as a possible determinant in grip strength, which is interesting as it had already been linked to muscle repair (Matteini et al., 2016). Generally the percentage of variance in population phenotypes explained by GWAS identified candidates is on the small side; however, the alignment between the biology of these genes and the traits they influence is undeniable, and the possibilities for new insights into the biology of disease incidence and progression using this unbiased approach are beyond that promised by any other type of analysis to date. As more genomic data become available for analysis we can expect further discoveries into the genetics of human aging using advanced computational methods to integrate across studies. These studies are expected to point to the causative agents in disease vulnerability, opening up studies into the underlying biology of these factors and how they interact with age and environment to determine longevity.
3. Caloric Restriction Studies
Since McCay's initial documentation (McCay et al., 1935), calorie restriction has endured 80 years of research to stand out as the most robust dietary intervention to extend average and maximum lifespan and delay the onset of age-related pathologies (Anderson and Weindruch, 2012). The effect of CR on longevity is conserved across a diverse range of species from unicellular organisms such as yeast to nematodes, invertebrates and mammals. To date a range of factors have been associated with the beneficial effects of CR, a small subset of which are discussed in the next section. As with any pursuit in medical research, confirmation of the translatability of beneficial outcomes established in studies of short-lived rodents species to humans is the litmus test. The translational gap from lab to clinics can be bridged by studies in nonhuman primates, which share a high degree of similarity to humans. Rhesus monkeys (Macaca mulatta) share 93% identity with humans at the genetic level (Zimin et al., 2014), and are highly similar in anatomy, physiology, and endocrinology (Colman and Anderson, 2011). Average lifespan for rhesus monkeys in captivity is ~ 26 years of age and the maximum lifespan reported nationally is ~ 40 years of age. Importantly incidence and prevalence of age-related diseases and the impact of age aligns nicely for humans and rhesus monkeys. Unlike rodents, rhesus monkeys display patterns of eating and sleeping behavior that mirror those of humans, and the aging trajectory is gradual, also like that of humans, beginning in middle age. In contrast to human studies, rhesus monkey studies can be designed to facilitate comprehensive monitoring of subjects and strict adherence to the study protocol. Given the high degree of translatability and the tractability in study design, nonhuman primates are a vital link between basic research and clinical application.
To investigate the translatability of CR's beneficial effects from rodents to primates, three independent rhesus monkey studies were initiated in the late 1980's. Two of these studies are ongoing: one at the National Institute on Aging (NIA) and the other at the Wisconsin National Primate Research Center based at the University of Wisconsin (UW)-Madison. The third study, performed at the University of Maryland reported favorable effects of CR, although the study was focused on obesity and glucoregulation with only a small cohort designated to CR (Bodkin et al., 2003). At the UW, the 25% restriction intervention in a cohort of 76 adult monkeys was associated with significant improvements in morbidity and mortality (Colman et al., 2009). These findings contrasted with the report from the parallel NIA study, where a difference in survival was not observed between groups within the cohort of 121 monkeys, although a trend toward lower morbidity was reported for CR monkeys compared to controls (Mattison et al., 2012). A subsequent report from UW suggested the gap between control and CR was not as great at NIA as for UW (Colman et al., 2014), indicating that comparisons between studies might paint a different picture as to the efficacy of CR in primates. This turned out to be the case (Mattison et al., 2017). Two major differences in study design included the timing of onset of CR and in the implementation of the diet. At UW CR was introduced in adults whereas at NIA CR was initiated separately in juveniles and advanced-age animals. CR did not confer a survival advantage in young onset animals; however, the old-onset NIA cohort, although not different between control and CR within the study, were long-lived compared to UW control fed monkeys. This was reflected in lower bodyweight, lower adiposity, and lower food intake, that for both control and CR paralleled the food intake of UW CR animals. The voluntary lower food intake of old-onset controls resulted in little separation between control and CR monkeys and yielded exceptionally long-lived monkeys. Six of the NIA old-set cohort lived to 40 years of age and one lived to 43 years of age, a record for rhesus monkeys in captivity. A common outcome of both studies was the significant delay in the incidence of age-related morbidity among CR animals. The take home message from this joint initiative is that CR delays aging in primates, where lower food intake is associated with improvements in health and survival. The implications of this work are broader, first that aging in primates can be manipulated, supporting the concept that aging is a valuable target for intervention and eventual clinical application, and second, that the mechanisms recruited by CR to impinge on aging will likely have utility in the development of treatments to delay or abrogate age-related disease vulnerability.
With evidence that CR is effective in long-lived species the next question is whether its beneficial effects and mechanistic underpinnings are conserved in humans. The hallmarks of mammalian CR include lower adiposity, increased insulin sensitivity, favorable lipid profiles, and increased levels of the adipose-derived hormone adiponectin. Short-term studies of CR in humans have been conducted as part of the multicenter study (CALERIE) in 2 phases. In the first phase of CALERIE studies (CALERIE-I), the metabolic effects of 6 or 12 months of CR was evaluated in overweight individuals with a target level of restriction of 20–30%. Favorable changes in body weight, body composition, glucoregulatory function and serum risk factors for cardiovascular disease were reported in CR individuals (Most et al., 2016). These outcomes were consistent with those reported for monkeys on CR (Edwards et al., 1998, Ramsey et al., 2000a), indicating species-conservation in the CR response. The second phase longitudinal CALERIE-II studies investigated the long-term (2 years) effects of 25% CR in healthy lean individuals. Results from CALERIE-II studies published so far indicate that the beneficial metabolic effects of CR observed in the 6-month CALERIE pilot studies are sustained with prolonged restriction in energy intake at 12 and 24 months. CR individuals displayed metabolic adaptation with reduced total daily energy expenditure at both 12 and 24 months and lowered resting metabolic rate after 12 months of CR (Ravussin et al., 2015). Reduction in metabolic rate has been previously linked to weight loss in humans (Kinney, 1995), and similar outcomes were reported for the early stages of the monkey CR study (Ramsey et al., 2000b) but were resolved over a longer time frame (Raman et al., 2007, Yamada et al., 2013) indicating this is likely another point of conservation in the CR response between humans and nonhuman primates. Unlike rodents on CR, circulating levels of IGF-1, cortisol, sex hormones and GH secretion were not altered in humans in both CALERIE clinical trials. Insufficient reduction in calorie intake (~ 11% CR versus the planned 25% CR) could explain some of the discrepancies on CR-induced metabolic changes observed in CALERIE compared with rodent studies; however there is also the possibility that these are species-specific differences in the CR response. IGF-1 and growth stimulating hormones did not differ between control and CR monkeys on 25% CR at UW (Ramsey et al., 2000b), and an independent study at University of Oregon reported no difference in sex hormones with CR implemented at a similar level (Sitzmann et al., 2010). Overall, these studies are highly suggestive that CR's effect on aging is translatable to humans and confirm that nonhuman primates do indeed bridge the gap between human and rodent studies.
4. Mechanistic Insights and Effector Proteins in Caloric Restriction
CR impinges on multiple signaling pathways that regulate growth, metabolism, oxidative stress response, damage repair, inflammation, autophagy, and proteostasis, to modulate the aging process (Lopez-Lluch and Navas, 2016). The relationship between calorie intake and longevity follows a U-shaped curve, dietary excess and malnutrition both negatively impact survival. Between the extremities there is an inverse linear relationship between lifespan and calorie/energy intake, suggesting that adaptive metabolism is a key component in the response to CR. We recently reported a conserved tissue-type independent transcriptional signature of CR in mice involving mitochondrial oxidative phosphorylation and redox metabolism pathways (Barger et al., 2015). This metabolic reprogramming is detected in other species on CR and is recapitulated in genetic models of enhanced longevity, supporting the concept that the CR-induced changes in energetics may indeed underlie the ability of the regimen to delay aging. Mitochondrial function has been linked to key age-sensitive processes including tissue rejuvenation and tumor suppression. Specifically, mitochondrial integrity is important for maintenance of pluripotency of stem cell populations (Xu et al., 2013) and multiple aspects of mitochondrial function have been implicated in cellular senescence (Correia-Melo et al., 2016, Korolchuk et al., 2017).
Studies in short-lived organisms from yeast to mice have given insight into the identity of potential “effectors” in the mechanism of delayed aging by CR. Many of the factors implicated in CR's mechanisms are involved in energy and nutrient sensing (Fig. 2) These include kinases and deacetylase enzymes involved in post translational modification, nutrient sensing mechanisms to direct cellular signaling, and transcription factors and co-activators that are required to launch a new program of metabolic balance. Several recent reviews have touched on longevity regulation and expand on evidence from rodent, fly, nematode, and yeast studies (Fontana and Partridge, 2015, Lopez-Lluch and Navas, 2016). Here will provide only a brief introduction to some of the most promising candidates in the mechanisms of CR and the next section describes interventions that have been proposed to target these regulatory nodes.
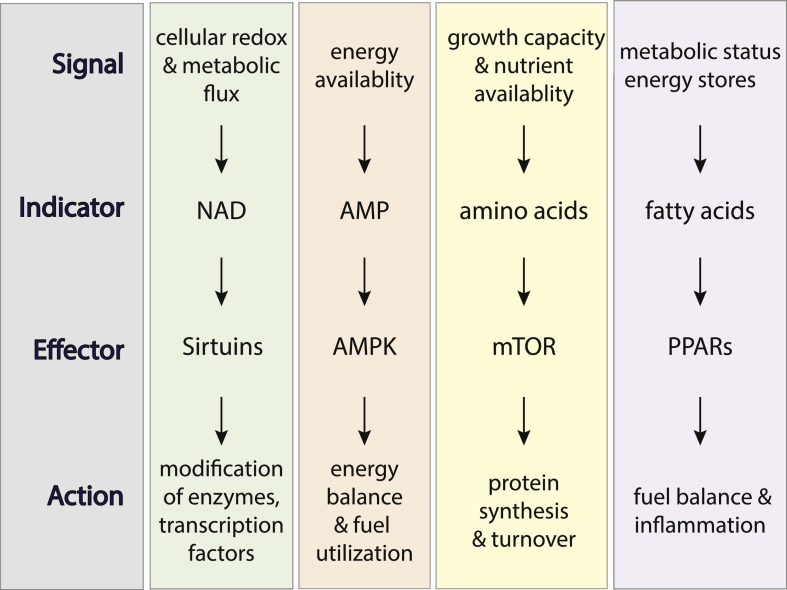
Fig. 2.
Metabolic regulators are among the strongest candidates as ‘effectors’ in the mechanisms of delayed aging with CR. These nutrient and energy sensing factors receive extracellular and intracellular signals via indicator molecules to implement various actions in pathways related to growth, metabolism, and inflammation.
AMP-activated protein kinase (AMPK) is a key intracellular signaling factor involved in the adaptive response to energy deficit or changes in energetic demand. AMPK senses energy availability and subsequently regulates other pathways including some of those implicated in aging such as mTOR (negative regulator) and SIRT1 (positive regulator) as outlined below. Aging is associated with a decline in AMPK inducibility (Reznick et al., 2007) whereas CR has been shown to activate the AMPK pathway in multiple tissue in animals (Canto and Auwerx, 2011). PGC-1a (peroxisome proliferator activated receptor gamma coactivator 1 alpha), is a transcriptional coactivator of nuclear receptor transcription factors such as PPARa and PPARg (Martinez-Redondo et al., 2015). This family of nuclear receptor transcription factors regulate genes involved in a wide spectrum of physiological functions including lipid metabolism and have been implicated in diabetes and metabolic syndrome (Semple et al., 2006). AMPK directly phosphorylates PGC-1a resulting in activation, thereby promoting the utilization of lipids as fuel. The mechanistic Target of Rapamycin (mTOR) is a nutrient sensing protein kinase that coordinates cellular growth and metabolism from nutrient and amino acid inputs (Laplante and Sabatini, 2012). mTOR exists in two complexes: mTORC1 and mTORC2 that impinge on protein synthesis, autophagy, and lipid metabolism (Kennedy and Lamming, 2016). Evidence suggests that the beneficial effects of CR on lifespan are at least in part dependent on mTORC1 signaling. Interestingly, AMPK is a negative regulator of mTOR, suggesting that there is a convergence of signaling under CR conditions to simultaneously lower growth signaling, through mTOR and related factors, and enhance lipid metabolism, through PGC-1a and PPARa. Sirtuins are a family of evolutionary conserved enzymes that influence a range of cellular activities including regulation of metabolism, cell fate determination, and chromatin remodeling (Bonkowski and Sinclair, 2016, Imai and Guarente, 2014). Most of the members of the sirtuin family remove acetyl groups to enhance activity of target proteins, although ADP-ribosylation activity has also been reported for some family members. Enzymatic activity of sirtuins requires nicotinamide adenine dinucleotide (NAD) as a co-substrate. This requirement intimately connects the sirtuin family to metabolism, as they are responsive to changes in NAD availability such as pathways of NAD regeneration and recycling and flux in redox metabolism. Taken together, these studies identify roles for nutrient sensitive metabolic regulators in the response to CR and point to the central importance of metabolic integrity in health and longevity.
5. Interventions that Harness CR's Mechanisms
Caloric restriction as an intervention is likely to be very difficult to implement in humans. Indeed the goal of CR research is to figure out how it works, not to promote it as a lifestyle. In order to gain the beneficial effects of CR without the restriction of calories, a number of nutraceuticals and established drugs are being explored as a means to mimic the effects of CR. The National Institute on Aging (NIA) at the National Institutes of Health has created the Interventions Testing Program (ITP) to investigate treatments with the potential to extend lifespan and delay age-related disease and dysfunction (Warner et al., 2000). To date several effective compounds have been identified some of which have been used in human clinical applications such as rapamycin (inhibitor of mTOR), metformin (activator of AMPK), and others that are only recently being applied in human studies such as resveratrol (activator of AMPK and SIRT1).
Resveratrol is a polyphenol found most notably in purple and red berries. The factor that is responsible for the metabolic effects of resveratrol is a point of debate, both AMPK and SIRT1 have been suggested to be the target of resveratrol (Lagouge et al., 2006, Um et al., 2010), and this has been the primary incentive to pursue its abilities as a CR mimetic (Selman, 2014). Resveratrol has been shown to extend lifespan in metabolically compromised mice (Baur et al., 2006). Evidence to support its ability to enhance longevity in non-obese metabolically healthy animals is lacking (Miller et al., 2011); however resveratrol has beneficial effects on age related pathologies (Pearson et al., 2008). The NIH database of clinical trials lists 129 studies involving resveratrol, 23 of which are currently open. Studies include outcomes such as cognitive function, cardiovascular health, and metabolic dysfunction secondary to obesity. It will be interesting to see if this natural compound makes its way into clinics.
Rapamycin was initially isolated from soil bacteria found on Easter Island or as it is locally known, Rapa Nui (Kennedy and Lamming, 2016). Rapamycin treatment causes inhibition of TORC I and has been shown to extend lifespan in a diverse number of species including yeast, worms, flies, and more recently mice (Harrison et al., 2009). Clinically, rapamycin has long been used as an immunosuppressant for prevention of organ transplant rejection; a function that is likely linked to its profound growth inhibitory effects. Use of rapamycin is limited however due to its adverse metabolic side effects. Recently several groups have conducted studies to optimize dose and frequency of rapamycin treatment with promising results (Arriola-Apelo et al., 2016, Bitto et al., 2016) to separate the longevity effects from the adverse metabolic side effects. In human studies only one report to date describes the use of rapamycin to counter an age-related deficiency. In this work a single dose of rapamycin improved immune parameters in aged individuals including the response to vaccination (Mannick et al., 2014). The NIH clinical trials database lists over 2000 studies involving rapamycin or derivatives thereof, most of which focus on cancer or immune-modulatory activities. Just over 400 are currently active; perhaps the time has come for additional studies of rapamycin treatment in humans that are focused on aging as an outcome.
Metformin is a drug that is commonly prescribed for the treatment of type 2 diabetes. Mechanistically, the major target of metformin is thought to be AMPK that is activated upon treatment (Lu et al., 2015, Zheng et al., 2012). Metformin treatment leads to a decrease in signaling through IGF/Insulin (Liu et al., 2011), and mTOR pathways (Kickstein et al., 2010, Nair et al., 2014). It is unclear at this time what the primary effect of metformin is, it has been proposed that changes in cell signaling are secondary to inhibition of Complex I in the mitochondrial electron transport chain (Foretz et al., 2014). The widespread use and long-term application of metformin in the clinical realm has resulted in thousands of publications. Many of these touch on diseases and disorders associated with aging. NIH clinical trails database lists 2000 studies of metformin treatment, 337 of which are currently active. Of particular interest is the TAME trail, “targeting aging with metformin”, which is the first of its kind in that aging itself is the process under investigation, with as emergence of comorbidity as the indexed outcome (Barzilai et al., 2016). The results of this study are highly anticipated as it paves the way for future clinical trials that focus on aging as the target for intervention, a goal that until recently was not possible through the US Food and Drug Administration.
6. Conclusions, Outstanding Questions, and Future Directions
Aging research has entered a very exciting period where traditional scientific approaches to understanding the biology of aging are converging with clinical research and epidemiology. Technological advances in the last few decades have brought aging research to a place that could not even have been imagined back in the days when the establishment of the National Institute on Aging first officially recognized the science of aging. We have already seen the identification of genes and biomarkers associated with healthy aging and exceptional aging, and studies in laboratory animals have laid out a rich framework of factors that have established roles in regulation of longevity.
Outstanding questions include the molecular basis for the role of energy metabolism in aging. How do differences in mitochondrial function create vulnerability to disease? How do defects in mitochondrial efficiency and adaptation arise? To what extent do minor differences in energetic capacity or fuel utilization influence other cellular functions? What networks within the cell are responsive to these relatively small age-related changes? Another important avenue of investigation is the role of lipid metabolism in aging and disease vulnerability. Changes in lipid handling are predicted to impact levels and composition of fatty acids that act as both intracellular and extracellular signaling moieties in addition to being the building blocks of membranes and other cellular structures. Lipid transport and lipid handling are common themes in human and laboratory aging studies, and differences in lipid metabolism have been strongly implicated in the mechanisms of CR, but how does this translate to a change in disease vulnerability as a function of age? Taking a broader view, it will be necessary to distinguish between events that are coincident with aging and those that are driving aging. Does aging arise first within discrete systems or is it orchestrated simultaneously across systems? To what extent are failures in individual processes such as rejuvenation and repair or induction of senescence responsible for age-related disease vulnerability?
To resolve these and other questions future directions must include synergistic collaborative efforts focused on aligning insights from human and laboratory aging studies. Development of advanced computational approaches such as meta-analyses and machine learning will enhance derivation of biological insights from large datasets. These insights can then be tested using genetic and pharmacological approaches in rodent studies to identify new effective interventions to delay aging. Extension of these studies to nonhuman primates will allow translation of biological insights. Caloric restriction research will also have a role to play, where interdisciplinary approaches can be brought to bear to determine the molecular details of CR's mechanisms and thereby identify the most promising candidate factors for targeted intervention (Fig. 3). There is tremendous interest among the public at large in discovering what influences the way we age and if the burden of age-related disease really is an inevitability of the aging process. Working together, researchers engaged in the multiple facets of aging biology are on track to have a profound impact on clinical approaches to healthy human aging.
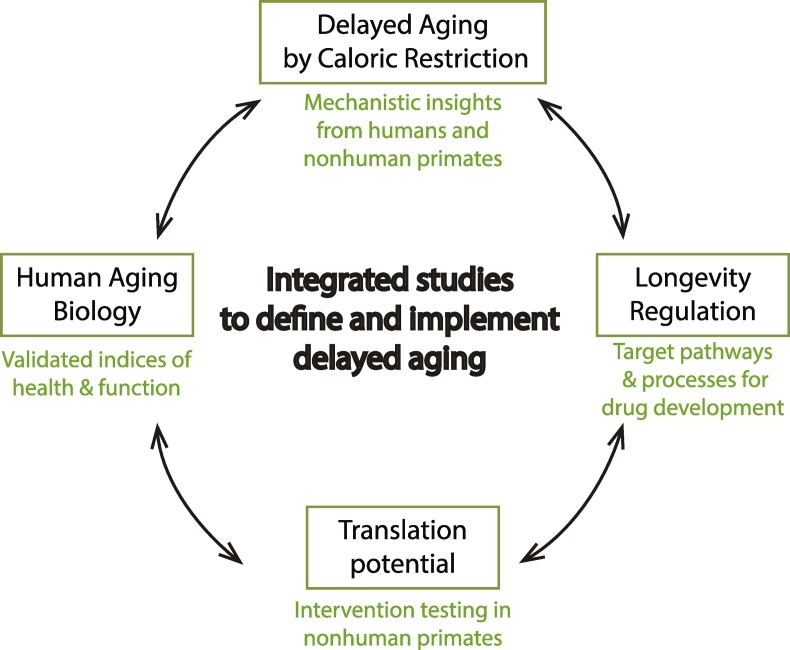
Fig. 3.
Proposed scheme for integrated studies to define and implement delayed aging. Caloric restriction (CR) provides mechanistic insights into signaling pathways and processes that regulate longevity. Factors and processes implicated in CR's mechanisms are very strong candidates for the development of drugs and treatments to enhance healthy aging in humans. These factors can be crosschecked against the growing body of work on human aging biology, and interventions developed from these avenues can be tested for translatability in nonhuman primate models before being proposed for human trials.
Search Strategy and Selection Criteria
Data for this Review were identified by searches of PubMed for relevant articles using the search terms “Aging, longitudinal studies, caloric restriction”. Additional searches were performed based on names of specific genes and pharmacological agents. Articles published in English between 1935 and 2017 were included in the search and results were prioritized for relevance. A limited selection of the articles and reviews identified are cited herein to comply with journal guidelines. We apologize to authors of the numerous excellent studies and review articles that had to be excluded.
Acknowledgments
This work was supported by NIH/NIA grants AG047358 and AG040178 and awards from the Glenn Foundation for Medical Research and the American Federation for Aging Research. The work was conducted with the use of resources and facilities at the William S Middleton Memorial Veterans Hospital, WI. The authors declare no conflict of interest.
References
- Andersen S.L., Sebastiani P., Dworkis D.A., Feldman L., Perls T.T. Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67A:395–405. doi: 10.1093/gerona/glr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.M., Weindruch R. The caloric restriction paradigm: implications for healthy human aging. Am. J. Hum. Biol. 2012;24:101–106. doi: 10.1002/ajhb.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi C.V., Malovini A., Roncarati R., Novelli V., Villa F., Condorelli G., Bellazzi R., Puca A.A. Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation Res. 2009;12:95–104. doi: 10.1089/rej.2008.0827. [DOI] [PubMed] [Google Scholar]
- Arriola-Apelo S.I., Pumper C.P., Baar E.L., Cummings N.E., Lamming D.W. Intermittent Administration of rapamycin extends the life span of female C57BL/6J mice. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:876–881. doi: 10.1093/gerona/glw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzmon G., Pollin T.I., Crandall J., Tanner K., Schechter C.B., Scherer P.E., Rincon M., Siegel G., Katz M., Lipton R.B. Adiponectin levels and genotype: a potential regulator of life span in humans. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:447–453. doi: 10.1093/gerona/63.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulchenko Y.S., Ripatti S., Lindqvist I., Boomsma D., Heid I.M., Pramstaller P.P., Penninx B.W., Janssens A.C., Wilson J.F., Spector T. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat. Genet. 2009;41:47–55. doi: 10.1038/ng.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger J.L., Anderson R.M., Newton M.A., da Silva C., Vann J.A., Pugh T.D., Someya S., Prolla T.A., Weindruch R. A conserved transcriptional signature of delayed aging and reduced disease vulnerability is partially mediated by SIRT3. PLoS One. 2015;10:e0120738. doi: 10.1371/journal.pone.0120738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N., Atzmon G., Schechter C., Schaefer E.J., Cupples A.L., Lipton R., Cheng S., Shuldiner A.R. Unique lipoprotein phenotype and genotype associated with exceptional longevity. JAMA. 2003;290:2030–2040. doi: 10.1001/jama.290.15.2030. [DOI] [PubMed] [Google Scholar]
- Barzilai N., Crandall J.P., Kritchevsky S.B., Espeland M.A. Metformin as a tool to target aging. Cell Metab. 2016;23:1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur J.A., Pearson K.J., Price N.L., Jamieson H.A., Lerin C., Kalra A., Prabhu V.V., Allard J.S., Lopez-Lluch G., Lewis K. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavers K.M., Hsu F.C., Houston D.K., Beavers D.P., Harris T.B., Hue T.F., Kim L.J., Koster A., Penninx B.W., Simonsick E.M. The role of metabolic syndrome, adiposity, and inflammation in physical performance in the health ABC study. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:617–623. doi: 10.1093/gerona/gls213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecham G.W., Martin E.R., Li Y.J., Slifer M.A., Gilbert J.R., Haines J.L., Pericak-Vance M.A. Genome-wide association study implicates a chromosome 12 risk locus for late-onset Alzheimer disease. Am. J. Hum. Genet. 2009;84:35–43. doi: 10.1016/j.ajhg.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Avraham D., Karasik D., Verghese J., Lunetta K.L., Smith J.A., Eicher J.D., Vered R., Deelen J., Arnold A.M., Buchman A.S. The complex genetics of gait speed: genome-wide meta-analysis approach. Aging (Albany NY) 2017;9:209–246. doi: 10.18632/aging.101151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitto A., Ito T.K., Pineda V.V., LeTexier N.J., Huang H.Z., Sutlief E., Tung H., Vizzini N., Chen B., Smith K. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. elife. 2016;5 doi: 10.7554/eLife.16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom D.E., Chatterji S., Kowal P., Lloyd-Sherlock P., McKee M., Rechel B., Rosenberg L., Smith J.P. Macroeconomic implications of population ageing and selected policy responses. Lancet. 2015;385:649–657. doi: 10.1016/S0140-6736(14)61464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin N.L., Alexander T.M., Ortmeyer H.K., Johnson E., Hansen B.C. Mortality and morbidity in laboratory-maintained rhesus monkeys and effects of long-term dietary restriction. J. Gerontol. A Biol. Sci. Med. Sci. 2003;58:212–219. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- Bonkowski M.S., Sinclair D.A. Slowing ageing by design: the rise of NAD + and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. (England) 2016:679–690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J.B., Augustine A.D., Frieden L.A., Hadley E., Howcroft T.K., Johnson R., Khalsa P.S., Kohanski R.A., Li X.L., Macchiarini F. Advances in geroscience: impact on healthspan and chronic disease. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69(Suppl. 1):S1–S3. doi: 10.1093/gerona/glu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C., Auwerx J. Calorie restriction: is AMPK a key sensor and effector? Physiology (Bethesda) 2011;26:214–224. doi: 10.1152/physiol.00010.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K., Doblhammer G., Rau R., Vaupel J.W. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collerton J., Barrass K., Bond J., Eccles M., Jagger C., James O., Martin-Ruiz C., Robinson L., von Zglinicki T., Kirkwood T. The Newcastle 85 + study: biological, clinical and psychosocial factors associated with healthy ageing: study protocol. BMC Geriatr. 2007;7:14. doi: 10.1186/1471-2318-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman R.J., Anderson R.M. Nonhuman primate calorie restriction. Antioxid. Redox Signal. 2011;14:229–239. doi: 10.1089/ars.2010.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman R.J., Anderson R.M., Johnson S.C., Kastman E.K., Kosmatka K.J., Beasley T.M., Allison D.B., Cruzen C., Simmons H.A., Kemnitz J.W. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman R.J., Beasley T.M., Kemnitz J.W., Johnson S.C., Weindruch R., Anderson R.M. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat. Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia-Melo C., Marques F.D., Anderson R., Hewitt G., Hewitt R., Cole J., Carroll B.M., Miwa S., Birch J., Merz A. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 2016;35:724–742. doi: 10.15252/embj.201592862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary I.J., Gow A.J., Pattie A., Starr J.M. Cohort profile: the Lothian birth cohorts of 1921 and 1936. Int. J. Epidemiol. 2012;41:1576–1584. doi: 10.1093/ije/dyr197. [DOI] [PubMed] [Google Scholar]
- Duchowny K.A., Peterson M.D., Clarke P.J. Cut points for clinical muscle weakness among older Americans. Am. J. Prev. Med. 2017 doi: 10.1016/j.amepre.2016.12.022. (pii: S0749-3797(16)307097, PMID:28190692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards I.J., Rudel L.L., Terry J.G., Kemnitz J.W., Weindruch R., Cefalu W.T. Caloric restriction in rhesus monkeys reduces low density lipoprotein interaction with arterial proteoglycans. J. Gerontol. A Biol. Sci. Med. Sci. 1998;53:B443–B448. doi: 10.1093/gerona/53a.6.b443. [DOI] [PubMed] [Google Scholar]
- Elliot A.J., Chapman B.P. Socioeconomic status, psychological resources, and inflammatory markers: results from the MIDUS study. Health Psychol. 2016;35:1205–1213. doi: 10.1037/hea0000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri E., Zoli M., Gonzalez-Freire M., Salive M.E., Studenski S.A., Ferrucci L. Aging and multimorbidity: new tasks, priorities, and frontiers for integrated gerontological and clinical research. J. Am. Med. Dir. Assoc. 2015;16:640–647. doi: 10.1016/j.jamda.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. The metabolic regulation of aging. Nat. Med. 2015;21:1416–1423. doi: 10.1038/nm.3998. [DOI] [PubMed] [Google Scholar]
- Fontana L., Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161:106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M., Guigas B., Bertrand L., Pollak M., Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- Franceschi C., Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69(Suppl. 1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K., Nadon N.L., Wilkinson J.E., Frenkel K., Carter C.S. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd P., Carr D., Roan C. Cohort profile: Wisconsin longitudinal study (WLS) Int. J. Epidemiol. 2014;43:34–41. doi: 10.1093/ije/dys194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S., Guarente L. NAD + and sirtuins in aging and disease. Trends Cell Biol. 2014;24:464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.C., Dong X., Vijg J., Suh Y. Genetic evidence for common pathways in human age-related diseases. Aging Cell. 2015;14:809–817. doi: 10.1111/acel.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy B.K., Lamming D.W. The mechanistic target of rapamycin: the grand ConducTOR of metabolism and aging. Cell Metab. 2016;23:990–1003. doi: 10.1016/j.cmet.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy B.K., Berger S.L., Brunet A., Campisi J., Cuervo A.M., Epel E.S., Franceschi C., Lithgow G.J., Morimoto R.I., Pessin J.E. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C.J. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kickstein E., Krauss S., Thornhill P., Rutschow D., Zeller R., Sharkey J., Williamson R., Fuchs M., Köhler A., Glossmann H. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc. Natl. Acad. Sci. U. S. A. 2010;107:21830–21835. doi: 10.1073/pnas.0912793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney J.M. Influence of altered body weight on energy expenditure. Nutr. Rev. 1995;53:265–268. doi: 10.1111/j.1753-4887.1995.tb05484.x. [DOI] [PubMed] [Google Scholar]
- Korolchuk V.I., Miwa S., Carroll B., von Zglinicki T. Mitochondria in cell senescence: is mitophagy the weakest link? EBioMedicine. 2017;21:6–12. doi: 10.1016/j.ebiom.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Fan Z., Edgerton S.M., Yang X., Lind S.E., Thor A.D. Potent anti-proliferative effects of metformin on trastuzumab-resistant breast cancer cells via inhibition of erbB2/IGF-1 receptor interactions. Cell Cycle. 2011;10:2959–2966. doi: 10.4161/cc.10.17.16359. [DOI] [PubMed] [Google Scholar]
- Locke A.E., Kahali B., Berndt S.I., Justice A.E., Pers T.H., Day F.R., Powell C., Vedantam S., Buchkovich M.L., Yang J. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo V.D., Antebi A., Bartke A., Barzilai N., Brown-Borg H.M., Caruso C., Curiel T.J., de Cabo R., Franceschi C., Gems D. Interventions to slow aging in humans: are we ready? Aging Cell. 2015;14:497–510. doi: 10.1111/acel.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lluch G., Navas P. Calorie restriction as an intervention in ageing. J. Physiol. 2016;594:2043–2060. doi: 10.1113/JP270543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Shi J., Li M., Gui B., Fu R., Yao G., Duan Z., Lv Z., Yang Y., Chen Z. Activation of AMPK by metformin inhibits TGF-β-induced collagen production in mouse renal fibroblasts. Life Sci. 2015;127:59–65. doi: 10.1016/j.lfs.2015.01.042. [DOI] [PubMed] [Google Scholar]
- Mannick J.B., Del Giudice G., Lattanzi M., Valiante N.M., Praestgaard J., Huang B., Lonetto M.A., Maecker H.T., Kovarik J., Carson S. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 2014;6:268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- Martinez-Redondo V., Pettersson A.T., Ruas J.L. The hitchhiker's guide to PGC-1alpha isoform structure and biological functions. Diabetologia. 2015;58:1969–1977. doi: 10.1007/s00125-015-3671-z. [DOI] [PubMed] [Google Scholar]
- Matteini A.M., Tanaka T., Karasik D., Atzmon G., Chou W.C., Eicher J.D., Johnson A.D., Arnold A.M., Callisaya M.L., Davies G. GWAS analysis of handgrip and lower body strength in older adults in the CHARGE consortium. Aging Cell. 2016;15:792–800. doi: 10.1111/acel.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison J.A., Roth G.S., Beasley T.M., Tilmont E.M., Handy A.M., Herbert R.L., Longo D.L., Allison D.B., Young J.E., Bryant M. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison J.A., Colman R.J., Beasley T.M., Allison D.B., Kemnitz J.W., Roth G.S., Ingram D.K., Weindruch R., de Cabo R., Anderson R.M. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 2017;8:14063. doi: 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCay C.M., Crowell M.F., Maynard L.A. The effect of retarded growth upon the length of life span and upon the ultimate body size. Nutrition. 1935;5:155–171. (discussion 172) [PubMed] [Google Scholar]
- Mezuk B., Lohman M.C., Rock A.K., Payne M.E. Trajectories of body mass indices and development of frailty: evidence from the health and retirement study. Obesity (Silver Spring) 2016;24:1643–1647. doi: 10.1002/oby.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.A., Harrison D.E., Astle C.M., Baur J.A., Boyd A.R., de Cabo R., Fernandez E., Flurkey K., Javors M.A., Nelson J.F. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R., Fabbri E., Khadeer M.A., Carlson O.D., Gonzalez-Freire M., Zhang P., Semba R.D., Ferrucci L. Plasma biomarkers of poor muscle quality in older men and women from the baltimore longitudinal study of aging. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:1266–1272. doi: 10.1093/gerona/glw046. (PMID: 27029859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most J., Tosti V., Redman L.M., Fontana L. Calorie restriction in humans: an update. Ageing Res. Rev. 2016 doi: 10.1016/j.arr.2016.08.005. (pii: S1568-1637(16)30183-0. PMID:27544442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair V., Sreevalsan S., Basha R., Abdelrahim M., Abudayyeh A., Rodrigues Hoffman A., Safe S. Mechanism of metformin-dependent inhibition of mammalian target of rapamycin (mTOR) and Ras activity in pancreatic cancer: role of specificity protein (Sp) transcription factors. J. Biol. Chem. 2014;289:27692–27701. doi: 10.1074/jbc.M114.592576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson K.J., Baur J.A., Lewis K.N., Peshkin L., Price N.L., Labinskyy N., Swindell W.R., Kamara D., Minor R.K., Perez E. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radler B.T. The midlife in the United States (MIDUS) series: a National Longitudinal Study of Health and Well-being. Open Health Data. 2014:2. doi: 10.5334/ohd.ai. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman A., Ramsey J.J., Kemnitz J.W., Baum S.T., Newton W., Colman R.J., Weindruch R., Beasley M.T., Schoeller D.A. Influences of calorie restriction and age on energy expenditure in the rhesus monkey. Am. J. Physiol. Endocrinol. Metab. 2007;292:E101–E106. doi: 10.1152/ajpendo.00127.2006. [DOI] [PubMed] [Google Scholar]
- Ramsey J.J., Colman R.J., Binkley N.C., Christensen J.D., Gresl T.A., Kemnitz J.W., Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp. Gerontol. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Ramsey J.J., Laatsch J.L., Kemnitz J.W. Age and gender differences in body composition, energy expenditure, and glucoregulation of adult rhesus monkeys. J. Med. Primatol. 2000;29:11–19. doi: 10.1034/j.1600-0684.2000.290102.x. [DOI] [PubMed] [Google Scholar]
- Ravussin E., Redman L.M., Rochon J., Das S.K., Fontana L., Kraus W.E., Romashkan S., Williamson D.A., Meydani S.N., Villareal D.T. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:1097–1104. doi: 10.1093/gerona/glv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick R.M., Zong H., Li J., Morino K., Moore I.K., Yu H.J., Liu Z.X., Dong J., Mustard K.J., Hawley S.A. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5:151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszkowska-Gancarz M., Bartoszewicz Z., Polosak J., Kurylowicz A., Jonas M., Mossakowska M., Franek E., Puzianowska-Kuznicka M. Total and high molecular weight adiponectin and level-modifying polymorphisms of ADIPOQ in centenarians. Endokrynol. Pol. 2012;63:439–446. [PubMed] [Google Scholar]
- Schachter F., Faure-Delanef L., Guenot F., Rouger H., Froguel P., Lesueur-Ginot L., Cohen D. Genetic associations with human longevity at the APOE and ACE loci. Nat. Genet. 1994;6:29–32. doi: 10.1038/ng0194-29. [DOI] [PubMed] [Google Scholar]
- Sebastiani P., Solovieff N., Dewan A.T., Walsh K.M., Puca A., Hartley S.W., Melista E., Andersen S., Dworkis D.A., Wilk J.B. Genetic signatures of exceptional longevity in humans. PLoS One. 2012;7:e29848. doi: 10.1371/journal.pone.0029848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiani P., Bae H., Sun F.X., Andersen S.L., Daw E.W., Malovini A., Kojima T., Hirose N., Schupf N., Puca A. Meta-analysis of genetic variants associated with human exceptional longevity. Aging (Albany NY) 2013;5:653–661. [Google Scholar]
- Selman C. Dietary restriction and the pursuit of effective mimetics. Proc. Nutr. Soc. 2014;73:260–270. doi: 10.1017/S0029665113003832. [DOI] [PubMed] [Google Scholar]
- Semple R.K.1, Chatterjee V.K., O'Rahilly S. PPAR gamma and human metabolic disease. J. Clin. Invest. 2006;116:581–589. doi: 10.1172/JCI28003. (PMID:16511590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shungin D., Winkler T.W., Croteau-Chonka D.C., Ferreira T., Locke A.E., Magi R., Strawbridge R.J., Pers T.H., Fischer K., Justice A.E. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187–196. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsick E.M., Newman A.B., Nevitt M.C., Kritchevsky S.B., Ferrucci L., Guralnik J.M., Harris T., Health A.B.C.S.G. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M644–M649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- Sitzmann B.D., Mattison J.A., Ingram D.K., Roth G.S., Ottinger M.A., Urbanski H.F. Impact of moderate calorie restriction on the reproductive neuroendocrine axis of male rhesus macaques. Open Longev. Sci. 2010;3:38–47. doi: 10.2174/1876326X00903010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um J.H., Park S.J., Kang H., Yang S., Foretz M., McBurney M.W., Kim M.K., Viollet B., Chung J.H. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkers D.J., Stek M.L., van der Mast R.C., de Craen A.J., Le Cessie S., Jolles J., Westendorp R.G., Gussekloo J. Generalized atherosclerosis, cognitive decline, and depressive symptoms in old age. Neurology. 2005;65:107–112. doi: 10.1212/01.wnl.0000167544.54228.95. [DOI] [PubMed] [Google Scholar]
- Warner H.R., Ingram D., Miller R.A., Nadon N.L., Richardson A.G. Program for testing biological interventions to promote healthy aging. Mech. Ageing Dev. (Ireland) 2000:199–207. doi: 10.1016/s0047-6374(00)00118-4. [DOI] [PubMed] [Google Scholar]
- Willcox B.J., Donlon T.A., He Q., Chen R., Grove J.S., Yano K., Masaki K.H., Willcox D.C., Rodriguez B., Curb J.D. FOXO3A genotype is strongly associated with human longevity. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox B.J., Willcox D.C., Suzuki M. Demographic, phenotypic, and genetic characteristics of centenarians in Okinawa and Japan: Part 1-centenarians in Okinawa. Mech. Ageing Dev. 2016 doi: 10.1016/j.mad.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Xu X., Duan S., Yi F., Ocampo A., Liu G.H., Izpisua Belmonte J.C. Mitochondrial regulation in pluripotent stem cells. Cell Metab. 2013;18:325–332. doi: 10.1016/j.cmet.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Colman R.J., Kemnitz J.W., Baum S.T., Anderson R.M., Weindruch R., Schoeller D.A. Long-term calorie restriction decreases metabolic cost of movement and prevents decrease of physical activity during aging in rhesus monkeys. Exp. Gerontol. 2013;48:1226–1235. doi: 10.1016/j.exger.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Chen H., Li J., Li T., Zheng B., Zheng Y., Jin H., He Y., Gu Q., Xu X. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes. 2012;61:217–228. doi: 10.2337/db11-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimin A.V., Cornish A.S., Maudhoo M.D., Gibbs R.M., Zhang X., Pandey S., Meehan D.T., Wipfler K., Bosinger S.E., Johnson Z.P. A new rhesus macaque assembly and annotation for next-generation sequencing analyses. Biol. Direct. 2014;9:20. doi: 10.1186/1745-6150-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]