Abstract
Background:
Duchenne muscular dystrophy (DMD) is caused by a defective gene located on the X-chromosome, responsible for the production of the dystrophin protein. Complications in the musculoskeletal system have been previously described in DMD patients. Whole body vibration exercise (WBVE) is a treatment that improves musculoskeletal function in movement disorders. The aim of this study was to review the effects of WBVE on functional mobility, bone and muscle in DMD patients.
Materials and Methods:
Four databases were searched. Three eligible studies were found; all three conclude the management of DMD patients with WBV was clinically well tolerated. The studies used a side-alternating WBV system, frequencies 7 - 24 Hz; and amplitudes 2 - 4 mm.
Results:
A work indicates that a temporary increase in creatine kinase in DMD during the first days of WBV was observed, but other authors did not find changes. No significant changes in bone mass, muscle strength or bone markers. Some patients reported subjective functional improvement during training. Interpretation:
Conclusion:
It is concluded that WBV seems to be a feasible and well tolerated exercise modality in DMD patients.
Keywords: whole body vibration exercise, Duchenne muscular dystrophy, rehabilitation
Introduction
Muscular dystrophy (MD) is an inherited group of degenerative disorders resulting from modifications in genes responsible for normal muscle function (Emery, 2002) and in which progressive muscle weakness is the main primary symptom (Flanigan, 2012). Duchenne muscular dystrophy (DMD) for example is caused by a defective gene located on the X-chromosome which is responsible for the production of a muscular protein, namely dystrophin (Hoffman, 1987;Ervasti,1990; Worton, 1995). This protein plays an important role in preventing muscle fatigue (Sander, 2000; Kobayashi, 2008; Allen et al. 2010). It has important functions related to cellular permeability of the sarcolemma(Rybakova, 2000; Goldstein, 2010) and protects dystrophin-associated proteins, including neuronal nitric oxide synthase (nNOS) (Lai, 2009). This in turn is important in the production of nitric oxide (NO) (Brenman, 1995) which regulates vasodilation and increases blood flow into muscle (Hellsten, 2012).
DMD affects only males (Strehle, 2015). Signs of myopathy may be detected from birth through laboratory findings although the clinical sign usually occurs later between two and three years of age(Gardner-Medwin, 1980; McDonald, 2012). Creatine kinase (CK) blood levels are often utilized as diagnostic markers for DMD (Cacchiarelli, 2011). Serum CK concentrations are elevated in these patients and usually exceed 1000 IU/L and can be as high as 30 000 IU/L (Strehle, 2015). CK levels however are a poor indicator of disease progression and are not routinely measured once the diagnosis has been established (Strehle, 2015). Mirski and Crawford (2014) have described that children with DMD might have different degrees of mild cognitive impairment or global developmental delay. However, they may also have average or above-average intelligence.
Muscle weakness and decreased muscle functioning affects normal bone growth and development. Back pain and fractures involving the long bones and vertebrae are frequently seen in boys with DMD due to decreased bone mineral density (BMD) (Morgenroth, 2012). In another study of 378 participants with DMD (ranging from one to 25 years of age), 21% were reported to have had fractures due to falls (McDonald, 2002). Another frequently reported secondary impairment in especially the non-ambulant child with DMD is scoliosis (Hsu, 2013; Canavese, 2014). The progressive weakness of the paraspinal muscles leads to trunk and body positional changes that facilitate the development of a progressive collapsing scoliosis. Scoliosis interferes with comfortable sitting and affects pulmonary function(Canavese, 2014). These children’s quality of life decreases over time and many suffer acute respiratory failure (Mangera, 2012).
Authors have reported that the optimum management of DMD requires a multidisciplinary approach and should focus on anticipatory and preventive measures as well as active interventions to address the primary and secondary aspects of the disease (Bushby 2010). Corticosteroids are also prescribed and have shown to prolong ambulation, however, given their effect on bone health may not be the best treatment intervention in this population (Morgenroth, 2012).
Exercise is a well-evidenced non-pharmacological treatment approach to improve bone mineral density (BMD) (Caulton, 2004). There is even some evidence to suggest that regular standing programs may have a positive effect on bone (Caulton, 2004). A Consensus Guideline document by Bushby et al. (2010) recommends that all boys with DMD who are ambulatory or in the early non-ambulatory stage participate in regular sub maximum exercise to avoid disuse muscle atrophy and other complications of inactivity. The guideline also recommends that low-resistance strength training and optimization of upper body function may provide additional benefits. Nevertheless, it also warns against over-activity as this can result in pain and myoglobinuria which in turn increases fatigue in the muscle (Bushby, 2010). Therapists and exercise professionals are thus challenged to find the delicate balance between activity and over-activity when designing exercise training programs for this population, and whole body vibration (WBV) could be a reliable option (Sanudo, 2010; Santos-Filho, 2012; Collado-Mateo, 2015).
WBV is increasingly being recognized as a useful mode for increasing strength and improving neuromuscular function (Sanudo, 2010;Santos-Filho, 2012; Collado-Mateo, 2015). WBV may be performed when a person either stands stationary or performs movements while standing, sitting or lying on an oscillating/vibratory platform (OVP) (Cochrane, 2010; Rittweger, 2010). The reported benefits of standing and/or exercising on an OVP include decreased joint compression, while still facilitating a contraction stronger than what is performed with the same exercise on dry-land (Rittweger, 2003; Cochrane, 2011; Boucher, 2015). A review published in 2010, however, pointed out that there is little scientific evidence supporting optimal vibration frequency and other treatment parameters to be used in the protocols involving WBV(Cochrane, 2010; Rittweger, 2010). Despite the lack of evidence this mode of exercise is increasingly being utilized in the DMD population (Soderpalm, 2013;Myers, 2014; Vry 2014). The use of WBV is increasing in populations with sub-optimal health such as, fibromyalgia (Sanudo, 2010; Collado-Mateo, 2015), cystic fibrosis (Roth, 2008; Maiworm, 2011), cerebral palsy (Sá-Caputo, 2015; Saquetto, 2015), osteogenesis imperfect (Semler, 2008) and multiple sclerosis (Jackson, 2008; Santos-Filho 2012). WBV exercise was firstly introduced (Hand, 2009) to try to reduce the effects of zero gravity experienced by astronauts while in space(LeBlanc, 2000).
The effect of the mechanical vibration on the musculoskeletal system would produce changes in the length of the muscle tendon complex. Due to this situation, a tonic muscle contraction via the tonic vibration reflex can be elicited (Rittweger, 2003;Cochrane 2011). Boucher et al. (2015) reported that acute soleus muscle vibration interferes with plantar flexion torque generation by distorting proprioceptive information. However, high-frequency vibration applied on soleus muscle elicited higher force reproduction errors than low-frequency stimulation. In addition, Cochrane, 2010 and Rittweger, 2010 have pointed out that there is little scientific documentation about the appropriate vibration frequency, well as other parameters to be used in WBV protocols. Further research is recommended according to the recommendation for reporting WBV intervention studies of the International Society of Musculoskeletal and Neuronal interactions (Rauch, 2010).
Besides the desirable clinical effect of WBV, undesirable side effects have also been reported. In untrained participants exposed to acute vibration Crewther et al. (2004) reported problems like, hot feet, itching of the lower limbs, vertigo and severe hip discomfort and Cronin et al. (2004) reported pain of jaw, neck and lower limbs from acute intermittent WBV. Monteleone et al. (2007) reported a case of significant morbidity following one session of WBV in a patient with asymptomatic nephrolithiasis and Franchignoni et al. (2013) described that a healthy elite athlete suffered two episodes of hematuria after WBV training.
Putting together the findings reported, considering the relevance (Caulton, 2004; Bushby, 2010; Rittweger, 2010; Sanudo, 2010; Soderpalm, 2013; Myers, 2014; Vry, 2014), the possible desirable effects of WBV(Crewther, 2004; Cronin, 2004; Monteleone, 2007; Franchignoni, 2013) and the possibility of the use of this kind of exercise to manage DMD patient, is presented this investigation. The aim of this study was to systematically appraise published research, in four databases, regarding the effects of exercise performed on a WBV on functional mobility, bone and muscle in DMD patients.
Materials and Methods
Search strategy
Three reviewers independently accessed bibliographical databases available through the Universidade do Estado do Rio de Janeiro. Searches were performed using PubMed, Scopus, Science Direct and PEDro databases on August, 2015 with the main keywords (a) “Whole Body vibration” and “Duchenne muscular dystrophy”, (b) “Duchenne muscular dystrophy”, (c) “muscular dystrophy”, (d) Exercise and “Duchenne muscular dystrophy”, (e) “physical activity” and “Duchenne muscular dystrophy” and (f) “Whole Body vibration”. The number of publications (NP) was determined to each item searched in each database. About the databases used, briefly, (a) PubMed comprises more than 24 million citations for biomedical literature from MEDLINE, life science journals and online book (http://www.ncbi.nlm.nih.gov/pubmed), (b) Science Direct is a leading full-text scientific database offering journal articles and book chapters from nearly 2500 journals and more than 30000 books (http://www.sciencedirect.com), (c) PEDro is the Physiotherapy Evidence Database and it is a free database of over 29000 randomised trials, systematic reviews and clinical practice guidelines in physiotherapy. PEDro is produced by the Centre for Evidence-Based Physiotherapy at The George Institute for Global Health. (http://www.pedro.org.au) and (d) Scopus is the largest abstract and citation database of peer-reviewed literature: scientific journals, books and conference proceedings. Delivering a comprehensive overview of the world’s research output in the fields of science, technology, medicine, social sciences, and arts and humanities (http://www.elsevier.com/online-tools/scopus).
As it is shown in Table 1, in general, in all the searches with selected keywords, the number of publications in the Science Direct database was higher than in the other databases. Nevertheless, the number of publications was the smallest in the PEDro database. The number of publications with the keywords “Duchenne Muscular Dystrophy” and “Muscular Dystrophy” is elevated. However, a small percentage of them although only is related to the WBV exercise independently on the database searched.
Table 1.
Number of publications in the PubMed, Scopus, Science Direct and PEDro databases
| Keywords | PubMed | Scopus | PEDro | Science Direct |
|---|---|---|---|---|
| “Whole Body vibration” and “Duchenne muscular dystrophy” | 3 | 4 | 0 | 119 |
| “Duchenne muscular dystrophy” | 7,881 | 11,825 | 22 | 11,583 |
| “muscular dystrophy” | 20,507 | 460 | 33 | 36,739 |
| Exercise and “Duchenne muscular dystrophy” | 317 | 460 | 5 | 3191 |
| "physical activity” and “Duchenne muscular dystrophy” | 29 | 83 | 0 | 702 |
| “Whole Body vibration” | 1,323 | 2,636 | 184 | 45,902 |
Inclusion criteria
To be included in this review, all studies had to investigate the effects of WBV in persons with DMD and had to comply with the following criteria: be a randomized controlled trial (RCT); in the absence of RCT’s, single group experimental studies were also considered (cross-over designs) and published in English. Studies were included if they enrolled participants with DMD who performed static or dynamic exercises on WBV. All three reviewers then read the 126 abstracts of studies deemed potentially eligible independently and for those that continued to meet the inclusion criteria the full texts were accessed. Where consensus could not be reached a fourth team member was consulted. Duplicates were eliminated.
The studies were independently appraised and the level of evidence of each one was classified according to the National Health and Medical Research Council hierarchy of evidence (NHMRC, 2009) (Merlin et al. 2009) (Figure 1). The methodological quality of the selected articles was evaluated following the PEDro scale (Morihisa, 2016). Briefly, each publication was evaluated according to: (a) eligibility criteria, (b) subjects were randomly allocated to groups, (c) concealed allocation, (d) the groups with baseline similarity, (e) blinding of the patients, (f) blinding of the therapists, (g) blinding of all assessors, (h) measures obtained from more than 85% of the subjects, (i) all subjects received the treatment or control condition or, at least one key outcome was analysed by “intention to treat”, (j) results of the groups with statistical comparisons and (h) point measures and measures of variability of outcome. Those publications with a score of seven or greater in the PEDro scale were considered of ‘high’ methodological quality, those with a score of five to six would be of ‘fair’ quality and a score of four or below were classified as ‘poor’ quality(Walser, 2009).
Figure 1.
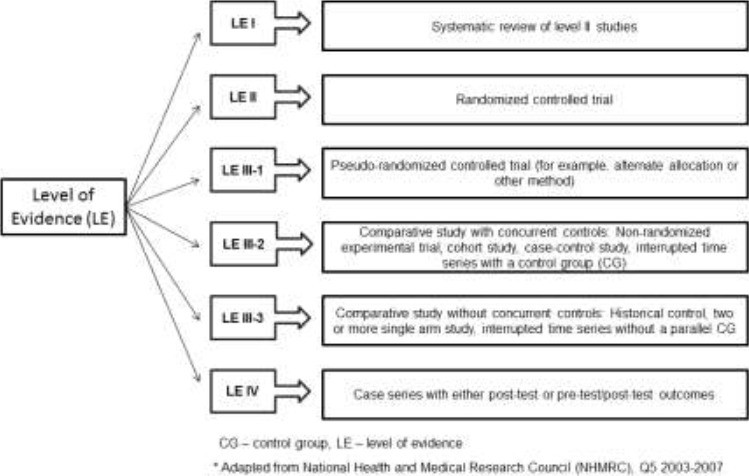
Level of Evidence adapted.
Exclusion criteria
Papers were excluded if they were (a) published in a language different from English; (b) review articles; (c) replies; (d) editorials; (e) abstracts; (f) proposal of a protocol; (g) books or chapter of books or abstracts; (h) animals studies; (i) including healthy people; (j) including combined treatments; (k) guidelines; (l) lectures; (m) occupational approaches; (n) in combination with other therapeutic procedures; and (o) patients with other diseases.
A flowchart, based in the PRISMA analysis(Liberati 2009), was done to show the steps in the selection of the full papers analyzed in this revision, as well as the elimination of unwanted publication. (Figure 2)
Figure 2.
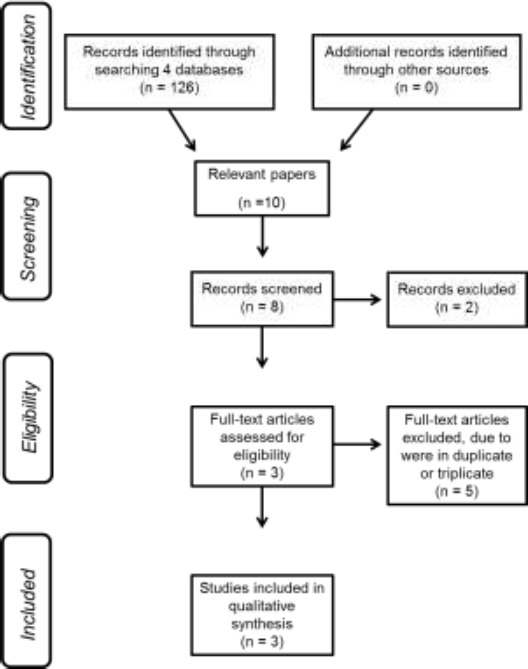
Flowchart indicating the steps to selected the full papers analysed in this revision.
Human Rights and Informed Consent
This article contains no studies with human subjects.
Data analysis
As data were not comparable, no statistical analysis was possible and findings are summarized in a narrative form.
Results
Figure 2 shows the search strategy results using PRISMA(Liberati 2009) to indicate how full papers were selected. One hundred twenty-six articles were found using the key words “Duchenne Muscular Dystrophy” and “whole body vibration”. Ten articles were screened and one hundred sixteen were excluded; reasons for exclusion: (a) not published in English, (b) reply, (c) including healthy persons, (d) including animal studies, (e) other techniques, (f) with combined techniques, (g) investigating occupational approaches, (h) editorials or letters, or (i) including other diseases. Three studies were finally included in this review. In table 2 depicts the type of platform, the subjects (number, sex, and age), the frequency and the amplitude used in the OVP used in these three studies. The level of evidence and the methodological quality is also shown.
Table 2.
Data on device of the oscillating/vibratory platform, number of subjects and frequency and amplitude or the peak to peak displacement of the vibration
| Reference | Level of evidence (LE) | PEDro Scale score (quality) | Platform Devices | Number of subjects/sex/age | Frequency(Hz) | Amplitude (mm)/PtPD (mm)(Rauch 2010) |
|---|---|---|---|---|---|---|
| Vry et al, 2014 | IV | 5 (fair) | side-alternating | n=22 (14 children with DMD/boys/mean 8.8 years and 8 children with SMA/5girls, 3boys/mean 9.9 years) | 15-18/ 18-24 | 4 |
| Soperpalm et al, 2013 | IV | 4 (poor) | side-alternating | n=6 (5.7 – 12.5 years, mean age of 6.8 years) | 16-24 | 2/4(PtPD) |
| Myers et al, 2014 | IV | 4 (poor) | side-alternating | n=4 (mean age of 10 years) | 7-20 | Not informed |
DMD – Duchenne muscular dystrophy, SMA – Spinal muscular atrophy, PtPD- peak-to-peak displacement.
All three studies included were Level of Evidence IV according to the NHMRC (Merlin 2009) and according to PEDro scale one study was of “fair” quality (score 5)(Vry 2014) and two were “poor” quality (score 4)(Soderpalm 2013, Myers 2014). The range of frequency of the vibrations generated in the OVP used was 7 to 24 Hz and amplitude was 2 to 4 mm. In one study the amplitude and the peak to peak displacement were not defined(Myers 2014). In total twenty four children with DMD participated in these investigations. Vry et al, 2014 (Vry 2014) studied twenty two patients, but only fourteen with DMD. The age varied from 4(Myers 2014) to 16 years(Vry 2014). All three studies used a side alternating OVP.
Table 3 shows the aim of the investigation, the protocol that was used, the findings and the conclusion of each analyzed full papers. Although, the investigations presented have aims with different approaches, all these papers present conclusions indicating the importance of WBV to improve and/or maintain clinical conditions that are relevant to the patient with DMD. Authors used different tools to evaluate the effect of WBV. Vry et al, 2014(Vry 2014) used the 6-min walking distance, 10 m walking time, time to climb 4 stairs, time to rise from supine, Medical Research Council Scale to examine muscle strength, myometry knee sum score, myometry elbow flexors, angle degree of dorsiflexion of the right ankle, and CK levels. Soderpalm et al, 2013, analyzed BMD and bone mineral content for total body, total hip and lumbar spine. Bone mass was performed using the dual-energy X-ray absorptiometry (DXA) and laser (DXL) Calscan technique. Biomarkers of bone and mineral metabolism (serum sclerostin, serum bone-specific alkaline phosphatase, serum osteocalcin insulin-like growth factor-I, insulin-like growth factor binding protein-3 and CK) were determined. Myers et al, 2014 measured the CK levels and functional mobility through North Star Ambulatory Assessment Score.
Table 3.
Aims, protocol, findings and conclusions identified in the selected papers involving DMD patients and WBV
| Reference | Aim | Protocol | Findings | Conclusion |
|---|---|---|---|---|
| Vry et al, 2014. | To evaluate the safety of WBV training in ambulatory children with DMD and SMA. | Two consecutive days with 2-3 training session per day in hospital. Between each training session, the patient had a break of at least 4 h. One training session consisted of 3 training units, each lasting 3 minutes, with a break of 3 minutes between each unit. After the teaching phase in the hospital, the patients continued with WBV training at home for 8 weeks (4 week with 15-18 Hz and 4 week with 18-20 Hz). They followed by another 4 weeks without training. Primary outcome was safety of the training, assessed clinically and by measuring serum CK levels. Secondary outcome was efficacy as measured by changes in time function test, muscle strength and angular degree of dorsiflexion of the ankles. | In boys with DMD, CK increased by 56% after the first day of training and returned to baseline after 8 weeks of continuous WBV training. No changes in laboratory parameters were observed in children with SMA. Secondary outcomes showed mild, but not significant improvements with the exception of the distance walked in the 6-min walking test in children with SMA, which rose from 371.3 m to 402.8 m (p < 0.01). | WBV training is clinically well tolerated in children with DMD and SMA. The relevance of the temporary increase in CK in DMD during the first days of training is unclear, but it is not related to clinical symptoms or deterioration. |
| Soperpalm et al, 2013. | To study the tolerability of WBV exercise in patients with DMD and its effects on muscle and bone. | WBV was performed two to three times a week for three month. Motor function, muscle strength, bone mass and biomarkers of bone and mineral metabolism were analyzed before and after the WBV period at 0, 3, 6 and 12 months. | No changes in CK activity were found, indicating that the WBV exercise did not further damage the skeletal muscle. No significant changes in bone mass, muscle strength or bone markers were found. There was a non-significant trend for the bone formation marker, bone-specific alkaline phosphate, to increase after three months of WBV. The bone formation marker levels returned to baseline three months after discontinuing WBV. | WBV therapy appears to be safe and well tolerated among ambulatory DMD patients. The potential benefits of WBV on bone and muscle in DMD remain to be elucidated. |
| Myers et al, 2014. | To evaluate the vibration therapy in patients with DMD. | All patients participated in a 4-week training period involving WBV sessions three time per week. Serum CK was measured, and adverse effects reviewed at each session with functional mobility assessed before and after the training period. | No major changes in functional mobility in the DMD patients were found. One patient had a transient increase in CK during the study; but, levels of this enzyme were stable when comparing the pre-training and post training values. Some patients reported subjective improvement during the training period. | Side-alternating vibration therapy is well tolerated in children with DMD and may have potential to improve or maintain functional mobility and strength in these patients. |
WBV - whole body vibration, DMD - Duchenne muscular dystrophy, SMA - spinal muscular atrophy, CK – creatine kinase.
Vry et al, 2014 have reported a temporary increase in CK in DMD during the first days of WBV. Nevertheless, Soperpalm et al, 2013 did not find changes in CK activity, and no significant changes in bone mass, muscle strength or bone markers were found. Myers et al, 2014 observed a transient increase in CK, and some patients reported subjective functional improvement during the training period. In all the publications(Soderpalm 2013, Myers 2014, Vry 2014) the management of DMD patient was considered clinically well tolerated.
Discussion
The relevance of WBV to treat patients with several diseases has been shown previously. However, it is observed that this modality of exercise is still poorly used to manage DMD patients, as verified by the small number of publications in four databases (PubMed, Scopus, Science Direct and PEDro).
The analysis of the information described in the selected full papers utilized in this investigation shows that WBV is considered clinically well tolerated in DMD patients and some patients reported subjective functional improvement.
An important number of publications involving “Duchenne muscular dystrophy” were found, but only a small percentage of these publications involves whole body vibration exercises independently. This finding is in agreement with Rittweger (2010), and Cochrane (2010) who pointed out that, although vibration exercise is broadly available, it seems that this exercise modality is still largely unknown to the scientific community.
The number of publications selected in this study involving the use of WBV exercise to treat patients with DMD was low, and their level of evidence were IV according to the NHMRC (Merlin, 2009). The methodological quality was “poor” or “fair” (Table 1). This is an important aspect that should make us call into question the obtained results is the methodological quality of the studies. Therefore, our findings demonstrate the necessity of further research with a higher methodological quality and level of evidence involving DMD and WBV exercise.
Mild exercise is recommended several times per week (ideally daily) for patients with DMD (Eagle, 2002; Bushby, 2010) in order to slow down the progress of the disease or to prevent its complications. WBV exercise, as a physical activity, seems to be safe and without impairment on the clinical condition of the DMD patient. The frequency of the vibrations generated of the OVP utilized was between 7 and 24 Hz. All authors of the investigations selected in this narrative revision (Soderpalm, 2013; Myers, 2014; Vry, 2014) have suggested that the conditions of the protocols are well tolerated by ambulatory patients with DMD. This fact would be highly relevant for the use of exercise in DMD rehabilitation without exacerbation of symptoms of the disease. Other authors have also shown that positive clinical findings after WBV have been obtained in patients with neuromuscular disease such as, (i) Parkinson with 3, 6 and 9 Hz (Arias, 2009; Chouza, 2011), or (ii) fibromyalgia with 12.5 Hz (Maiworm, 2011).
Despite these encouraging findings reported in this current revision, the effects should be interpreted with caution due to the variability of the frequencies (between 7 and 24Hz). Future research should be performed to examine the dose-response relationship and optimal dosing regimen of WBV according to several other parameters, such as exercise type, status training on vibration training, and the use of external load during vibration exercise. Additional focus on frequency and peak-to-peak displacement of the mechanical stimulus would also aid in the elaboration of appropriate protocols.
The current study has some limitations that must be considered in the interpretation of the findings. Firstly, it was found a small number of publications involving WBV and DMD patients. Furthermore, caution should be taken when generalizing the results due to the methodological variations concerning the biomechanical parameters or the variability of the protocols used. In addition, although we tried to retrieve the articles involving WBV and DMD with the selected keywords, it is not sure that all studies on this topic have been identified, including articles that were not published in English and articles published in journals that were not indexed in the databases there were searched. In addition, the limited number of publications with high methodological quality (RCTs) must also be considered and this fact could of course affect the evidence of the findings. Therefore, studies with a higher methodological quality would be desirable. The clinical application of WBV to the patients is that it is especially not harmful and is safe for rehabilitation.
In conclusion, despite the limitations, it can be verified that the use of WBV exercise has proven to be safe and could be an important option in the rehabilitation of patients with DMD. Moreover, benefits, without clinical complication to the patient with DMD have been reported. WBV seems to be a feasible and well tolerated modality exercise modality to manage DMD patients.
Acknowledgments
The authors thank the Brazilian Government agencies (CNPq, FAPERJ) and UERJ for the support.
References
- Allen D. G, Zhang B. T, Whitehead N. P. “Stretch-induced membrane damage in muscle:comparison of wild-type and mdx mice.”. Adv Exp Med Biol. 2010;682:297–313. doi: 10.1007/978-1-4419-6366-6_17. [DOI] [PubMed] [Google Scholar]
- Arias P, Chouza M, Vivas J, Cudeiro J. “Effect of whole body vibration in Parkinson’s disease:a controlled study.”. Mov Disord. 2009;24(6):891–898. doi: 10.1002/mds.22468. [DOI] [PubMed] [Google Scholar]
- Boucher J. A, Normand M. C, Boisseau E, Descarreaux M. “Sensorimotor control during peripheral muscle vibration:an experimental study.”. J Manipulative Physiol Ther. 2015;38(1):35–43. doi: 10.1016/j.jmpt.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Brenman J. E, Chao D. S, Xia H, Aldape K, Bredt D. S. “Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy.”. Cell. 1995;82(5):743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- Bushby K, Finkel R, Birnkrant D. J, Case L. E, Clemens P. R, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, Poysky J, Shapiro F, Tomezsko J, Constantin C D. M. D. C. C. W. Group. “Diagnosis and management of Duchenne muscular dystrophy, part 2:implementation of multidisciplinary care.”. Lancet Neurol. 2010;9(2):177–189. doi: 10.1016/S1474-4422(09)70272-8. [DOI] [PubMed] [Google Scholar]
- Cacchiarelli D, Legnini I, Martone J, Cazzella V, D’Amico A, Bertini E, Bozzoni I. “miRNAs as serum biomarkers for Duchenne muscular dystrophy.”. EMBO Mol Med. 2011;3(5):258–265. doi: 10.1002/emmm.201100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavese F, Rousset M, Le Gledic B, Samba A, Dimeglio A. “Surgical advances in the treatment of neuromuscular scoliosis.”. World J Orthop. 2014;5(2):124–133. doi: 10.5312/wjo.v5.i2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulton J. M, Ward K. A, Alsop C. W, Dunn G, Adams J. E, Mughal M. Z. “A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy.”. Arch Dis Child. 2004;89(2):131–135. doi: 10.1136/adc.2002.009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouza M, Arias P, Vinas S, Cudeiro J. “Acute effects of whole-body vibration at 3, 6, and 9 hz on balance and gait in patients with Parkinson’s disease.”. Mov Disord. 2011;26(5):920–921. doi: 10.1002/mds.23582. [DOI] [PubMed] [Google Scholar]
- Cochrane D. J. “Vibration exercise:the potential benefits.”. Int J Sports Med. 2011;32(2):75–99. doi: 10.1055/s-0030-1268010. [DOI] [PubMed] [Google Scholar]
- Cochrane D. J, Stannard S. R, Firth E. C, Rittweger J. “Comparing muscle temperature during static and dynamic squatting with and without whole-body vibration.”. Clin Physiol Funct Imaging. 2010;30(4):223–229. doi: 10.1111/j.1475-097X.2010.00931.x. [DOI] [PubMed] [Google Scholar]
- Collado-Mateo D, Adsuar J. C, Olivares P. R, Del Pozo-Cruz B, Parraca J. A, Del Pozo-Cruz J, Gusi N. “Effects of Whole-Body Vibration Therapy in Patients with Fibromyalgia:A Systematic Literature Review.”. Evid Based Complement Alternat Med. 2015;2015:719082. doi: 10.1155/2015/719082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crewther B, Cronin J, Keogh J. “Gravitational forces and whole body vibration:implications for prescription of vibratory stimulation.”. Physical Therapy in Sport. 2004;5(1):37–43. [Google Scholar]
- Cronin J, Crewther B. “Training volume and strength and power development.”. J Sci Med Sport. 2004;7(2):144–155. doi: 10.1016/s1440-2440(04)80004-5. [DOI] [PubMed] [Google Scholar]
- Eagle M. “Report on the muscular dystrophy campaign workshop:exercise in neuromuscular diseases Newcastle, January 2002.”. Neuromuscul Disord. 2002;12(10):975–983. doi: 10.1016/s0960-8966(02)00136-0. [DOI] [PubMed] [Google Scholar]
- Emery A. E. “The muscular dystrophies.”. Lancet. 2002;359(9307):687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M, Ohlendieck K, Kahl S. D, Gaver M. G, Campbell K. P. “Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle.”. Nature. 1990;345(6273):315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- Flanigan K. M. “The muscular dystrophies.”. Semin Neurol. 2012;32(3):255–263. doi: 10.1055/s-0032-1329199. [DOI] [PubMed] [Google Scholar]
- Franchignoni F, Vercelli S, Ozcakar L. “Hematuria in a runner after treatment with whole body vibration:a case report.”. Scand J Med Sci Sports. 2013;23(3):383–385. doi: 10.1111/j.1600-0838.2012.01478.x. [DOI] [PubMed] [Google Scholar]
- Gardner-Medwin D. “Clinical features and classification of the muscular dystrophies.”. Br Med Bull. 1980;36(2):109–115. doi: 10.1093/oxfordjournals.bmb.a071623. [DOI] [PubMed] [Google Scholar]
- Goldstein J. A, McNally E. M. “Mechanisms of muscle weakness in muscular dystrophy.”. J Gen Physiol. 2010;136(1):29–34. doi: 10.1085/jgp.201010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand J, Verscheure S, Osternig L. “A comparison of whole-body vibration and resistance training on total work in the rotator cuff.”. J Athl Train. 2009;44(5):469–474. doi: 10.4085/1062-6050-44.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten Y, Nyberg M, Jensen L. G, Mortensen S. P. “Vasodilator interactions in skeletal muscle blood flow regulation.”. J Physiol. 2012;590(Pt 24):6297–6305. doi: 10.1113/jphysiol.2012.240762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. P, Brown R. H, Kunkel L. M., Jr “Dystrophin:the protein product of the Duchenne muscular dystrophy locus.”. Cell. 1987;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hsu J. D, Quinlivan R. “Scoliosis in Duchenne muscular dystrophy (DMD).”. Neuromuscul Disord. 2013;23(8):611–617. doi: 10.1016/j.nmd.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Jackson K. J, Merriman H. L, Vanderburgh P. M, Brahler C. J. “Acute effects of whole-body vibration on lower extremity muscle performance in persons with multiple sclerosis.”. J Neurol Phys Ther. 2008;32(4):171–176. doi: 10.1097/NPT.0b013e31818ee760. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y. M, Rader E. P, Crawford R. W, Iyengar N. K, Thedens D. R, Faulkner J. A, Parikh S. V, Weiss R. M, Chamberlain J. S, Moore S. A, Campbell K. P. “Sarcolemma-localized nNOS is required to maintain activity after mild exercise.”. Nature. 2008;456(7221):511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Thomas G. D, Yue Y, Yang H. T, Li D, Long C, Judge L, Bostick B, Chamberlain J. S, Terjung R. L, Duan D. “Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy.”. J Clin Invest. 2009;119(3):624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc A, Schneider V, Shackelford L, West S, Oganov V, Bakulin A, Voronin L. “Bone mineral and lean tissue loss after long duration space flight.”. J Musculoskelet Neuronal Interact. 2000;1(2):157–160. [PubMed] [Google Scholar]
- Liberati A, Altman D. G, Tetzlaff J, Mulrow C, Gotzsche P. C, Ioannidis J. P, Clarke M, Devereaux P. J, Kleijnen J, Moher D. “The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions:explanation and elaboration.”. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiworm A. I, Monteiro M. B, Santos-Filho S. D, Lopes A. J, Azeredo L, Missailidis S, Marín P. J, Bernardo-Filho M. “Cystic fibrosis and the relevance of the whole-body vibration exercises in oscillating platforms:a short review.”. Health. 2011;03(10):656–662. [Google Scholar]
- Mangera Z, Panesar G, Makker H. “Practical approach to management of respiratory complications in neurological disorders.”. Int J Gen Med. 2012;5:255–263. doi: 10.2147/IJGM.S26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C. M. “Clinical approach to the diagnostic evaluation of hereditary and acquired neuromuscular diseases.”. Phys Med Rehabil Clin N Am. 2012;23(3):495–563. doi: 10.1016/j.pmr.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D. G, Kinali M, Gallagher A. C, Mercuri E, Muntoni F, Roper H, Jardine P, Jones D. H, Pike M. G. “Fracture prevalence in Duchenne muscular dystrophy.”. Dev Med Child Neurol. 2002;44(10):695–698. doi: 10.1017/s0012162201002778. [DOI] [PubMed] [Google Scholar]
- Merlin T, Weston A, Tooher R. “Extending an evidence hierarchy to include topics other than treatment:revising the Australian ’levels of evidence’.”. BMC Med Res Methodol. 2009;9:34. doi: 10.1186/1471-2288-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirski K. T, Crawford T. O. “Motor and cognitive delay in Duchenne muscular dystrophy:implication for early diagnosis.”. J Pediatr. 2014;165(5):1008–1010. doi: 10.1016/j.jpeds.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Monteleone G, De Lorenzo A, Sgroi M, De Angelis S, Di Renzo L. “Contraindications for whole body vibration training:a case of nephrolitiasis.”. J Sports Med Phys Fitness. 2007;47(4):443–445. [PubMed] [Google Scholar]
- Morgenroth V. H, Hache L. P, Clemens P. R. “Insights into bone health in Duchenne muscular dystrophy.”. Bonekey Rep. 2012;1:9. doi: 10.1038/bonekey.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihisa R, Eskew J, McNamara A, Young J. “Dry Needling in Subjects with Muscular Trigger Points in the Lower Quarter:A Systematic Review.”. Int J Sports Phys Ther. 2016;11(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- Myers K. A, Ramage B, Khan A, Mah J. K. “Vibration therapy tolerated in children with Duchenne muscular dystrophy:a pilot study.”. Pediatr Neurol. 2014;51(1):126–129. doi: 10.1016/j.pediatrneurol.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Rauch F, Sievanen H, Boonen S, Cardinale M, Degens H, Felsenberg D, Roth J, Schoenau E, Verschueren S, Rittweger JM International Society of and I Neuronal. “Reporting whole-body vibration intervention studies:recommendations of the International Society of Musculoskeletal and Neuronal Interactions.”. J Musculoskelet Neuronal Interact. 2010;10(3):193–198. [PubMed] [Google Scholar]
- Rittweger J. “Vibration as an exercise modality:how it may work, and what its potential might be.”. Eur J Appl Physiol. 2010;108(5):877–904. doi: 10.1007/s00421-009-1303-3. [DOI] [PubMed] [Google Scholar]
- Rittweger J, Mutschelknauss M, Felsenberg D. “Acute changes in neuromuscular excitability after exhaustive whole body vibration exercise as compared to exhaustion by squatting exercise.”. Clin Physiol Funct Imaging. 2003;23(2):81–86. doi: 10.1046/j.1475-097x.2003.00473.x. [DOI] [PubMed] [Google Scholar]
- Roth J, Wust M, Rawer R, Schnabel D, Armbrecht G, Beller G, Rembitzki I, Wahn U, Felsenberg D, Staab D. “Whole body vibration in cystic fibrosis--a pilot study.”. J Musculoskelet Neuronal Interact. 2008;8(2):179–187. [PubMed] [Google Scholar]
- Rybakova I. N, Patel J. R, Ervasti J. M. “The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin.”. J Cell Biol. 2000;150(5):1209–1214. doi: 10.1083/jcb.150.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá-Caputo D. C, Costa-Cavalcanti R, Carvalho-Lima R. P, Arnobio A, Bernardo R. M, Ronikeile-Costa P, Kutter C, Giehl P. M, Asad N. R, Paiva D. N, Pereira H. V, Unger M, Marin P. J, Bernardo-Filho M. “Systematic review of whole body vibration exercises in the treatment of cerebral palsy:Brief report.”. Dev Neurorehabil. 2015:1–7. doi: 10.3109/17518423.2014.994713. [DOI] [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Harris S. A, Iannaccone S. T, Stull J. T, Thomas G. D, Victor R. G. “Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy.”. Proc Natl Acad Sci U S A. 2000;97(25):13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Filho S. D, Cameron M. H, Bernardo-Filho M. “Benefits of whole-body vibration with an oscillating platform for people with multiple sclerosis:a systematic review.”. Mult Scler Int. 2012;2012:274728. doi: 10.1155/2012/274728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanudo B, de Hoyo M, Carrasco L, McVeigh J. G, Corral J, Cabeza R, Rodriguez C, Oliva A. “The effect of 6-week exercise programme and whole body vibration on strength and quality of life in women with fibromyalgia:a randomised study.”. Clin Exp Rheumatol. 2010;28(6 Suppl)(63):S40–45. [PubMed] [Google Scholar]
- Saquetto M, Carvalho V, Silva C, Conceicao C, Gomes-Neto M. “The effects of whole body vibration on mobility and balance in children with cerebral palsy:a systematic review with meta-analysis.”. J Musculoskelet Neuronal Interact. 2015;15(2):137–144. [PMC free article] [PubMed] [Google Scholar]
- Semler O, Fricke O, Vezyroglou K, Stark C, Stabrey A, Schoenau E. “Results of a prospective pilot trial on mobility after whole body vibration in children and adolescents with osteogenesis imperfecta.”. Clin Rehabil. 2008;22(5):387–394. doi: 10.1177/0269215507080763. [DOI] [PubMed] [Google Scholar]
- Soderpalm A. C, Kroksmark A. K, Magnusson P, Karlsson J, Tulinius M, Swolin-Eide D. “Whole body vibration therapy in patients with Duchenne muscular dystrophy--a prospective observational study.”. J Musculoskelet Neuronal Interact. 2013;13(1):13–18. [PubMed] [Google Scholar]
- Strehle E. M, Straub V. “Recent advances in the management of Duchenne muscular dystrophy.”. Arch Dis Child. 2015 doi: 10.1136/archdischild-2014-307962. [DOI] [PubMed] [Google Scholar]
- Vry J, Schubert I. J, Semler O, Haug V, Schonau E, Kirschner J. “Whole-body vibration training in children with Duchenne muscular dystrophy and spinal muscular atrophy.”. Eur J Paediatr Neurol. 2014;18(2):140–149. doi: 10.1016/j.ejpn.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Walser R. F, Meserve B. B, Boucher T. R. “The effectiveness of thoracic spine manipulation for the management of musculoskeletal conditions:a systematic review and meta-analysis of randomized clinical trials.”. J Man Manip Ther. 2009;17(4):237–246. doi: 10.1179/106698109791352085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worton R. “Muscular dystrophies:diseases of the dystrophin-glycoprotein complex.”. Science. 1995;270(5237):755–756. doi: 10.1126/science.270.5237.755. [DOI] [PubMed] [Google Scholar]


