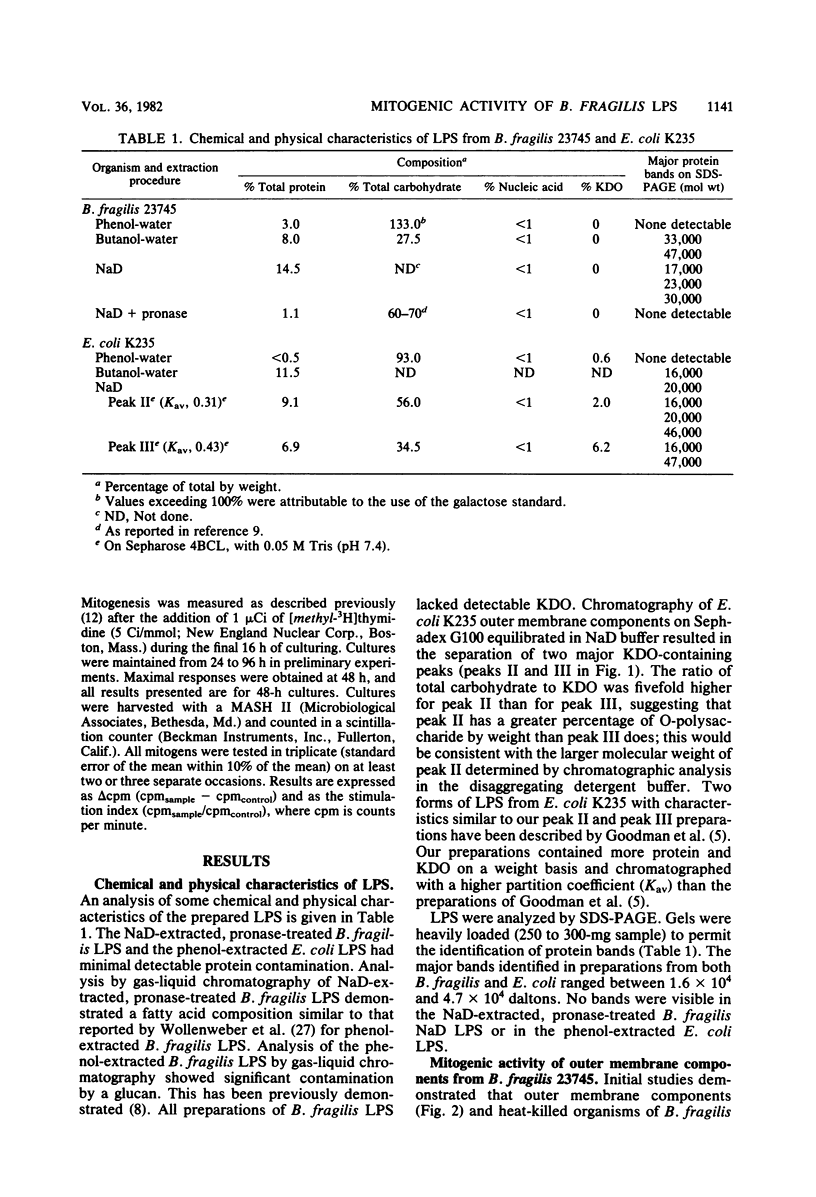
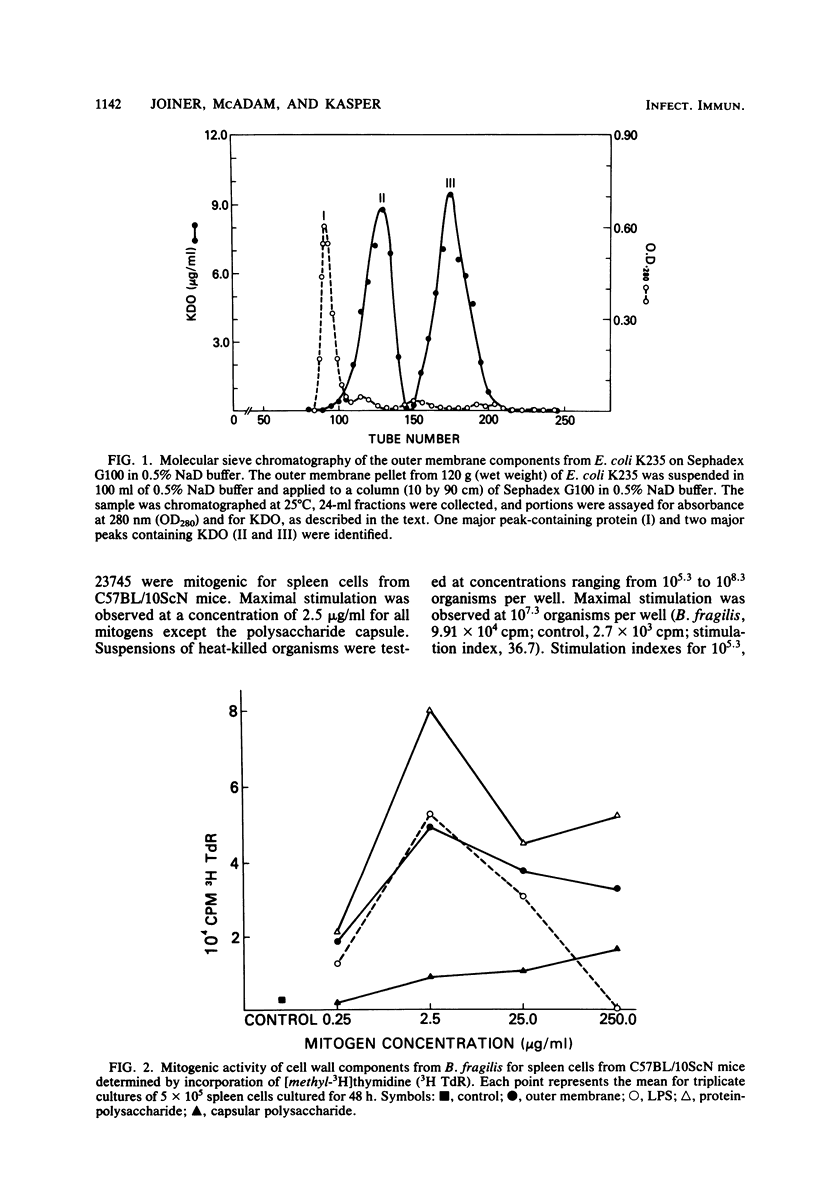
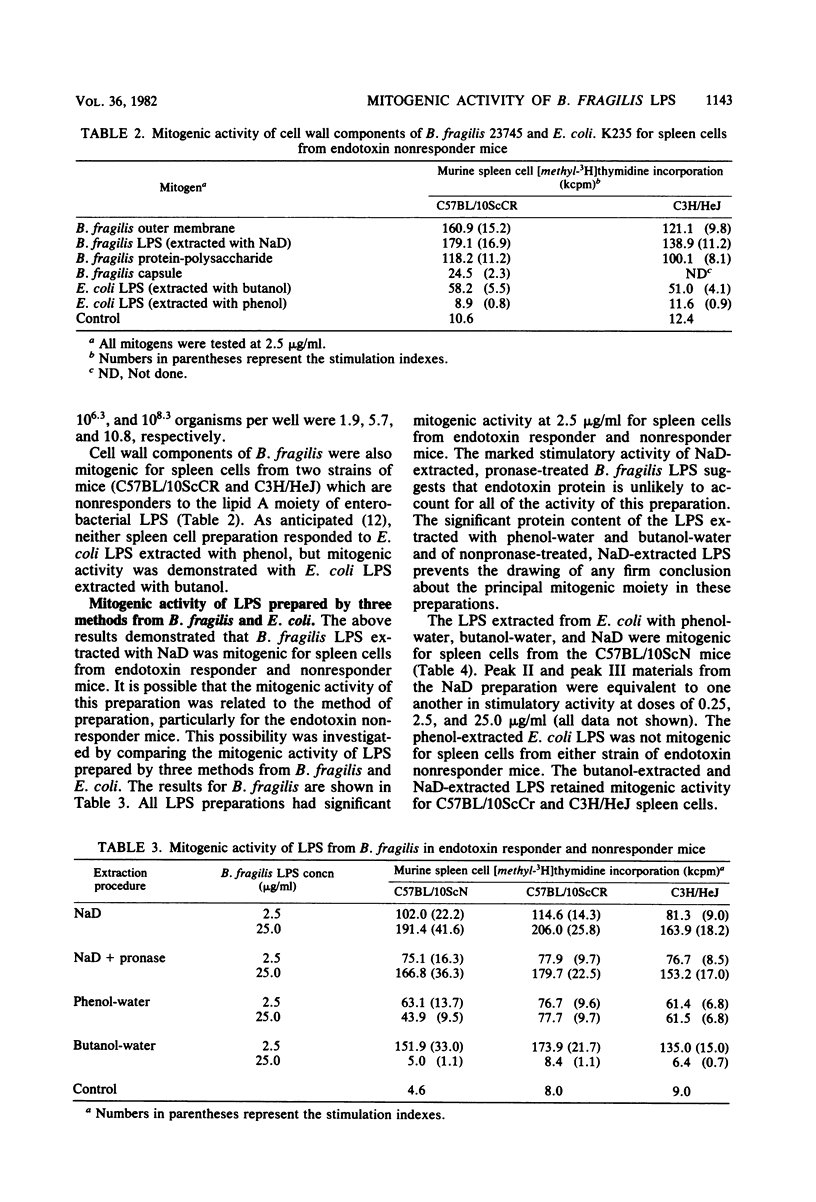
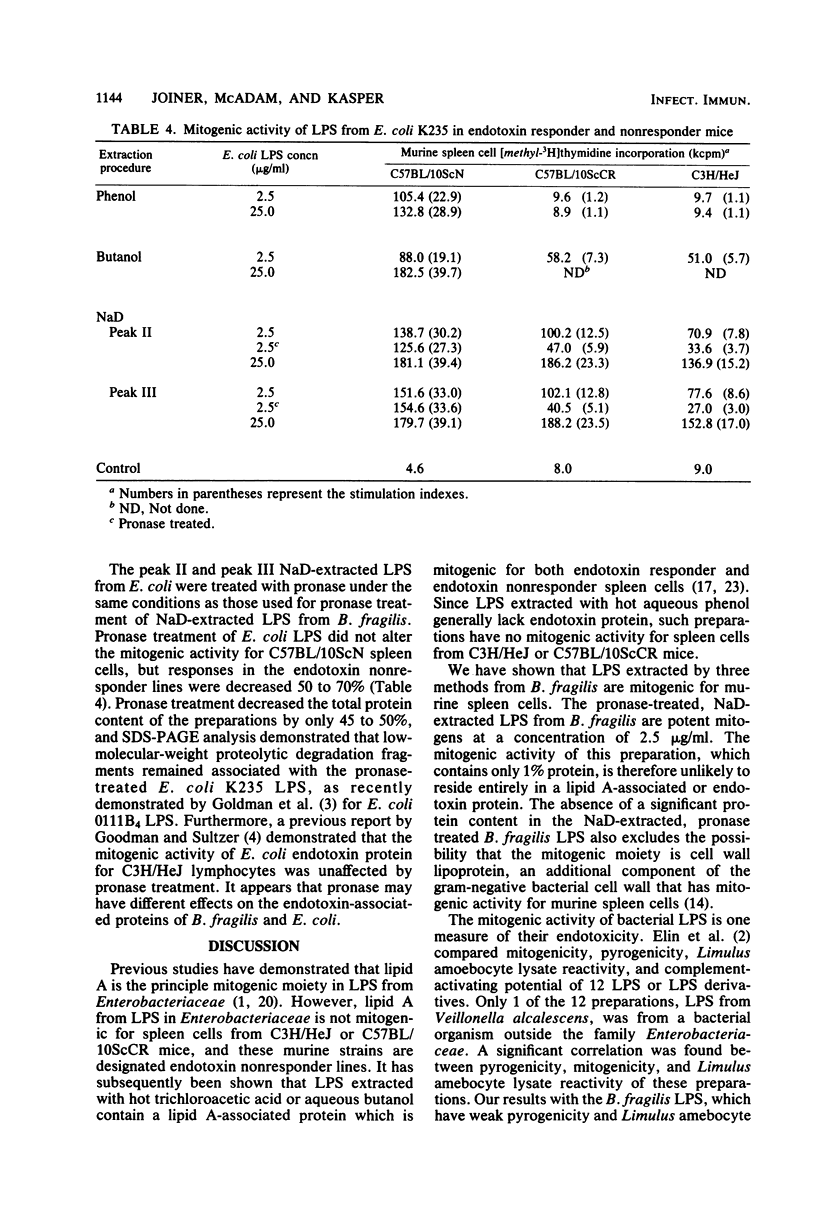
Abstract
The lipopolysaccharides (LPS) from Bacteroides fragilis are structurally atypical and give weak responses in most tests of endotoxic activity, but the mitogenic activity of LPS from B. fragilis has not been tested. We prepared LPS from B. fragilis 23745 by three methods and compared their mitogenic activity for murine spleen cells with that of LPS from Escherichia coli K235 prepared by similar techniques. LPS extracted from B. fragilis with hot phenol-water, with butanol-water, or by detergent separation from the outer membrane were mitogenic for spleen cells from C57BL/10ScN, C57BL/10ScCR, and C3H/HeJ mice. The outer membrane, the outer membrane protein-polysaccharide complex, and the capsular polysaccharide from B. fragilis were also mitogenic for spleen cells from the same murine strains. LPS extracted from E. coli K235 with hot phenol-water, butanol-water, or sodium deoxycholate were mitogenic for C57BL/10ScN spleen cells, but only the LPS extracted with butanol and deoxycholate were stimulatory for spleen cells from C57BL/10ScCR and C3H/HeJ mice. Two types of LPS varying in the 2-keto-3-deoxyoctonate-to-carbohydrate ratio were isolated from E. coli K235 with sodium deoxycholate; both endotoxins contained protein which was typical of lipid A or endotoxin protein. These results indicate that the LPS from B. fragilis is a potent mitogen for spleen cells from endotoxin responder and endotoxin nonresponder mice.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiller J. M., Skidmore B. J., Morrison D. C., Weigle W. O. Relationship of the structure of bacterial lipopolysaccharides to its function in mitogenesis and adjuvanticity. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2129–2133. doi: 10.1073/pnas.70.7.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elin R. J., Sandberg A. L., Rosentreich D. L. Comparison of the pyrogenicity, Limulus activity mitogenicity and complement reactivity of several bacterial endotoxins and related compounds. J Immunol. 1976 Oct;117(4):1238–1242. [PubMed] [Google Scholar]
- Goldman R. C., White D., Leive L. Identification of outer membrane proteins, including known lymphocyte mitogens, as the endotoxin protein of Escherichia coli 0111. J Immunol. 1981 Oct;127(4):1290–1294. [PubMed] [Google Scholar]
- Goodman G. W., Sultzer B. M. Characterization of the chemical and physical properties of a novel B-lymphocyte activator, endotoxin protein. Infect Immun. 1979 Jun;24(3):685–696. doi: 10.1128/iai.24.3.685-696.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M. G., Morrison D. C., Weigle W. O. Modulation of lipopolysaccharide (LPS)-mediated function by structural differences of two physically distinct fractions of Escherichia coli K235 LPS. J Immunol. 1977 May;118(5):1852–1857. [PubMed] [Google Scholar]
- Kasper D. L. Chemical and biological characterization of the lipopolysaccharide of Bacteroides fragilis subspecies fragilis. J Infect Dis. 1976 Jul;134(1):59–66. doi: 10.1093/infdis/134.1.59. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Onderdonk A. B., Reinap B. G., Linberg A. A. Variations of Bacteroides fragilis with in vitro passage: presence of an outer membrane-associated glycan and loss of capsular antigen. J Infect Dis. 1980 Nov;142(5):750–756. doi: 10.1093/infdis/142.5.750. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Seiler M. W. Immunochemical characterization of the outer membrane complex of Bacteroides fragilis subspecies fragilis. J Infect Dis. 1975 Oct;132(4):440–450. doi: 10.1093/infdis/132.4.440. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Lehmann V., Mayer H., Rietschel E. T., Weckesser J. Chemical structure and biological activities of lipid A's from various bacterial families. Naturwissenschaften. 1978 Nov;65(11):578–585. doi: 10.1007/BF00364907. [DOI] [PubMed] [Google Scholar]
- McAdam K. P., Ryan J. L. C57BL/10/CR mice: nonresponders to activation by the lipid a moiety of bacterial lipopolysaccharide. J Immunol. 1978 Jan;120(1):249–253. [PubMed] [Google Scholar]
- McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967 Aug;6(8):2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- Melchers F., Braun V., Galanos C. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J Exp Med. 1975 Aug 1;142(2):473–482. doi: 10.1084/jem.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Berman D. T. Brucella abortus lipopolysaccharide is mitogenic for spleen cells of endotoxin-resistant C3H/HeJ mice. J Immunol. 1979 Dec;123(6):2915–2919. [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Betz S. J., Jacobs D. M. Isolation of a lipid A bound polypeptide responsible for "LPS-initiated" mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976 Sep 1;144(3):840–846. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy D. L., Shands J. W., Jr, Adler W. H., Smith R. T. Mitogenicity of bacterial endotoxins: characterization of the mitogenic principle. J Immunol. 1973 Aug;111(2):352–357. [PubMed] [Google Scholar]
- Pier G. B., Markham R. B., Eardley D. Correlation of the biologic responses of C3H/HEJ mice to endotoxin with the chemical and structural properties of the lipopolysaccharides from Pseudomonas aeruginosa and Escherichia coli. J Immunol. 1981 Jul;127(1):184–191. [PubMed] [Google Scholar]
- Sultzer B. M., Goodman G. W. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ lymphocytes. J Exp Med. 1976 Sep 1;144(3):821–827. doi: 10.1084/jem.144.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Westphal O. Bacterial endotoxins. The second Carl Prausnitz Memorial Lecture. Int Arch Allergy Appl Immunol. 1975;49(1-2):1–43. [PubMed] [Google Scholar]
- Wollenweber H. W., Rietschel E. T., Hofstad T., Weintraub A., Lindberg A. A. Nature, type of linkage, quantity, and absolute configuration of (3-hydroxy) fatty acids in lipopolysaccharides from Bacteroides fragilis NCTC 9343 and related strains. J Bacteriol. 1980 Dec;144(3):898–903. doi: 10.1128/jb.144.3.898-903.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


