Abstract
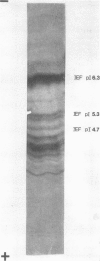
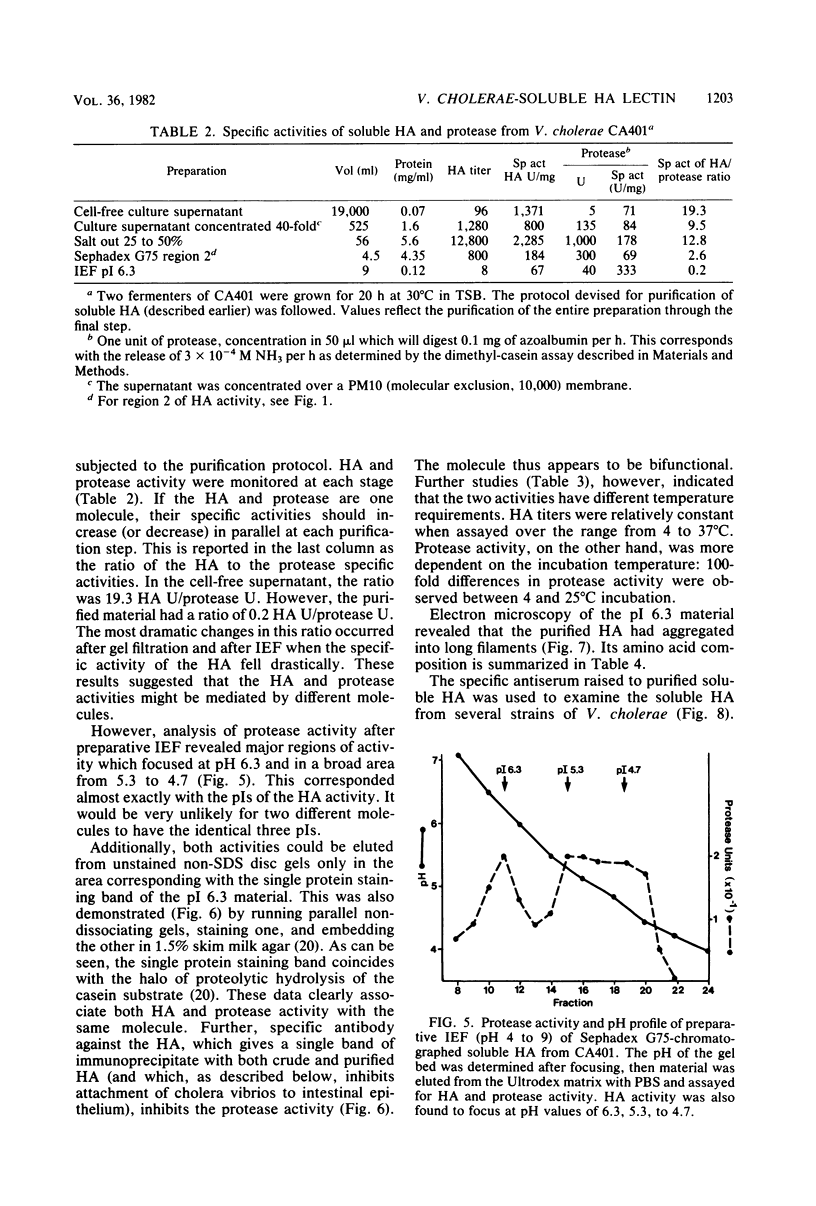
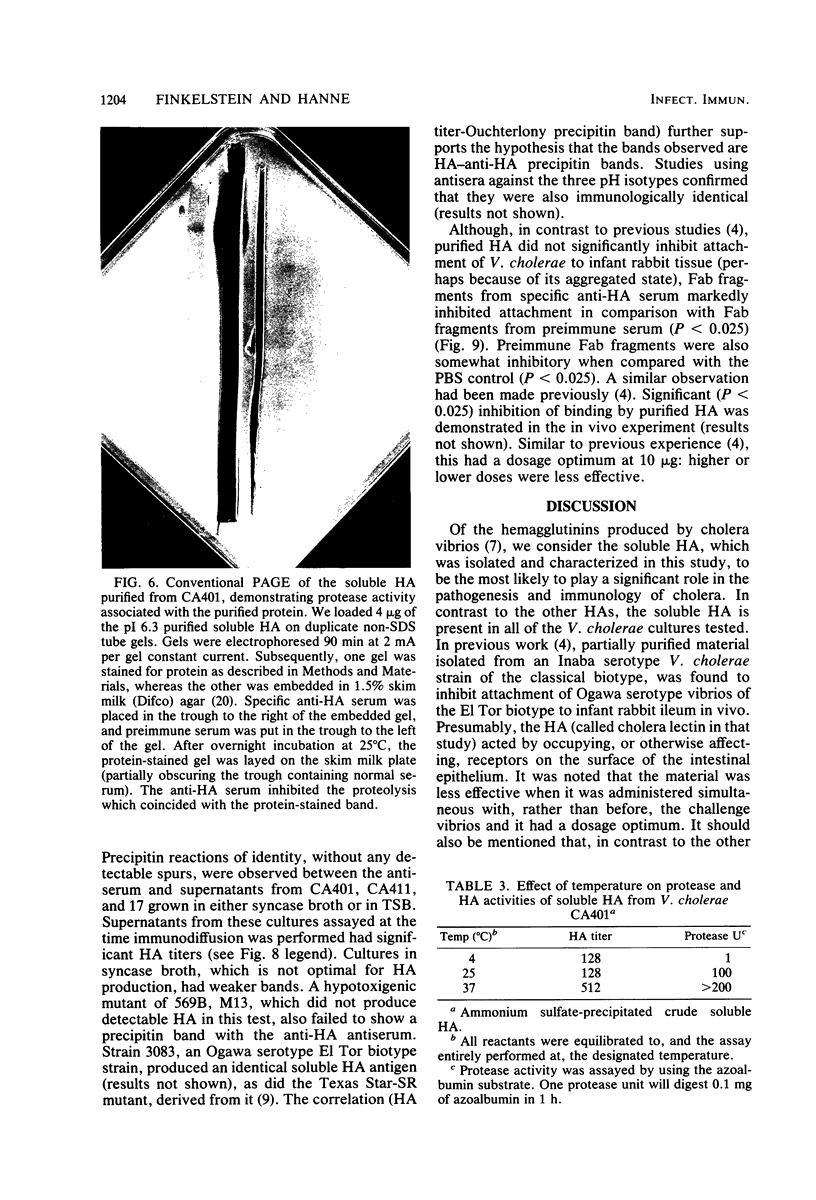
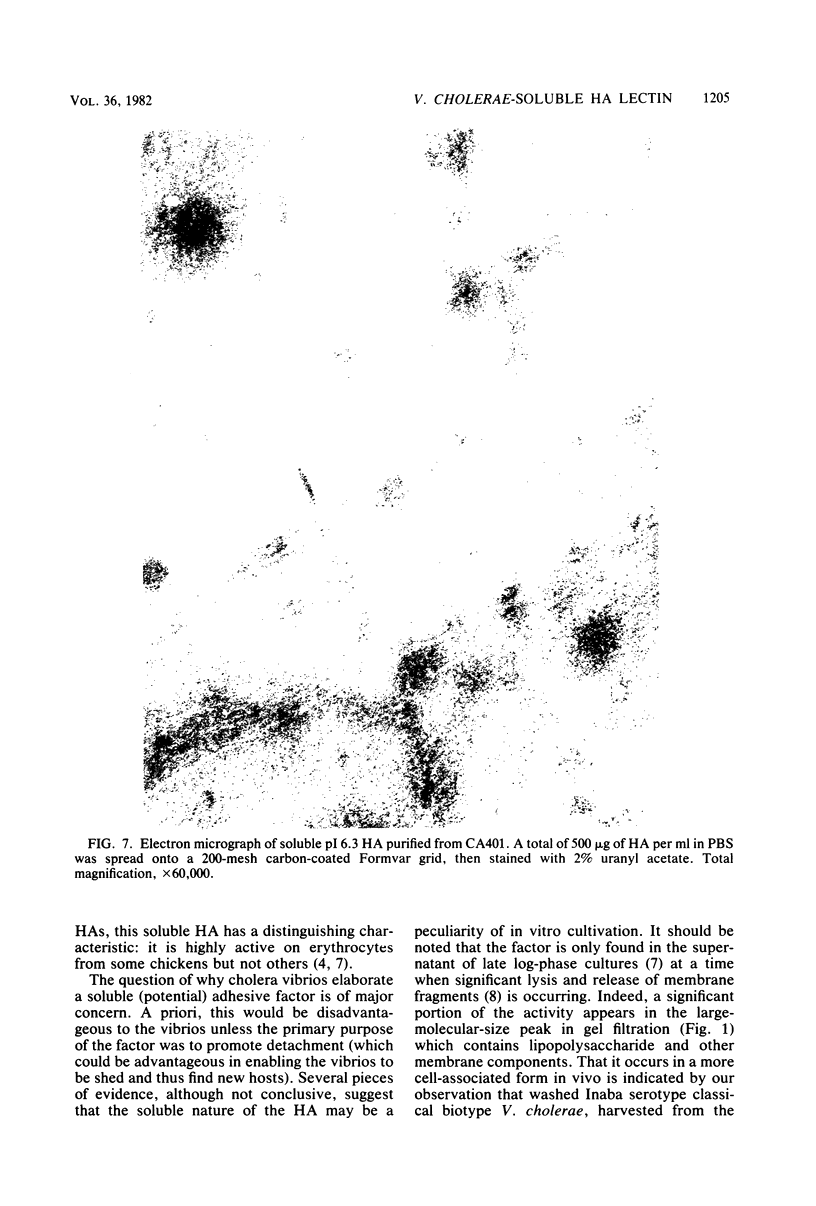
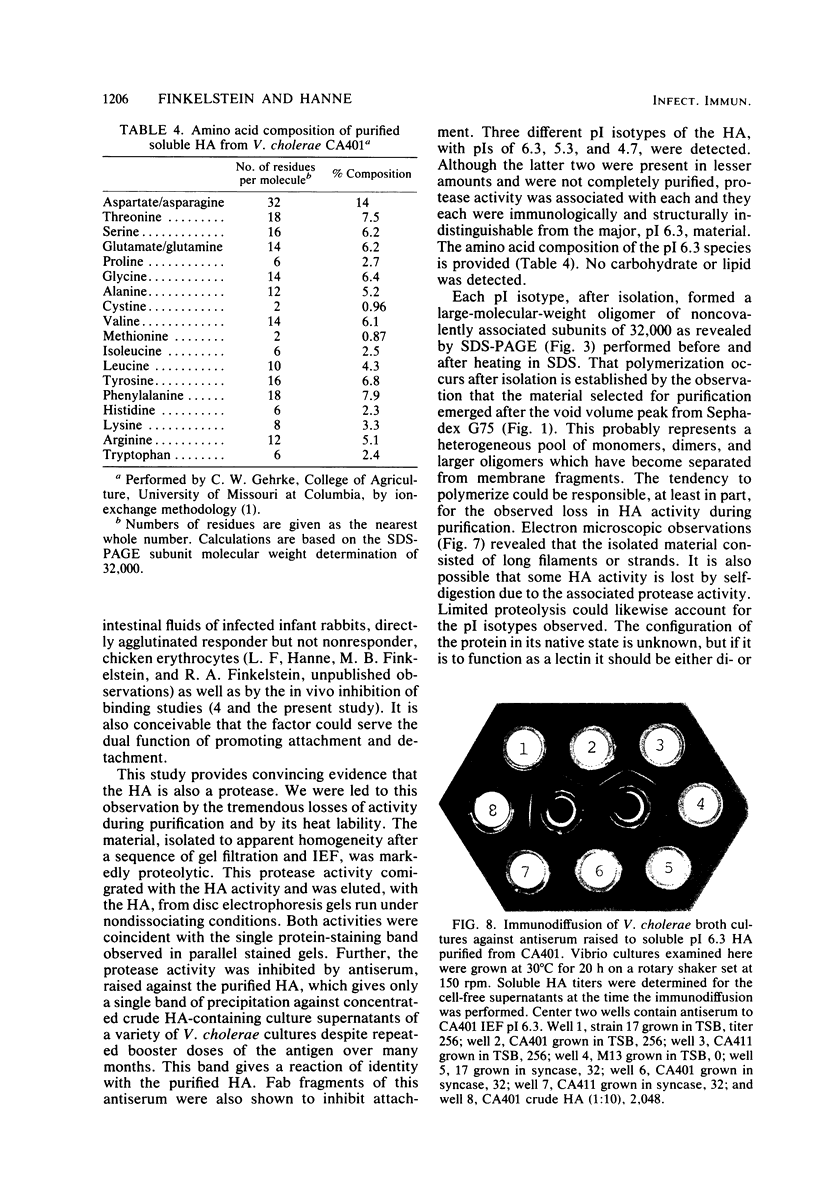
The soluble hemagglutinin (HA) (cholera lectin) produced by Vibrio cholerae strain CA401 was purified to apparent homogeneity by a sequence of ammonium sulfate fractionation, gel filtration, and preparative isoelectric focusing. Soluble HA activity was found to focus at three different pIs, 6.3, 5.3, and 4.7. Each of the factors migrated as a large-molecular-weight protein in sodium dodecyl sulfate-polyacrylamide gel electrophoresis under normal conditions, and each, upon heating in sodium dodecyl sulfate was found to dissociate into 32,000-molecular-weight subunits. Treating the samples with a reducing agent did not affect their mobility. Each gave a reaction of immunological identity with antiserum prepared against the others. Thus, there are apparently three distinct pH isotypes of soluble HA which exist as noncovalently associated polymers of 32,000-molecular-weight subunits. Electron microscopy of purified preparations revealed long filamentous polymers. The molecule does not stain as a glycoprotein; it is hydrophobic; it is inactivated during incubation at 25, 37, or 60 degrees C; and it has significant protease activity. The protease activity likewise focused at pH values of 6.3 and 5.3 to 4.7, and it was inhibited by antiserum against the HA. However, whereas the HA is active at 4 degrees C, the protease is not. The soluble HA is, therefore, a bifunctional molecule capable of mediating hemagglutination and proteolysis. Its amino acid composition is reported. Fab fragments of antibody against the purified HA inhibited attachment of heterologous serotype-biotype V. cholerae to infant rabbit small bowel.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Allweiss B., O'Brien P. C., Halstead S. A., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vitro studies. Infect Immun. 1981 Oct;34(1):241–249. doi: 10.1128/iai.34.1.241-249.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanne L. F., Finkelstein R. A. Characterization and distribution of the hemagglutinins produced by Vibrio cholerae. Infect Immun. 1982 Apr;36(1):209–214. doi: 10.1128/iai.36.1.209-214.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D., van der Laan J. W., de Leij L., Witholt B. Release of outer membrane fragments from normally growing Escherichia coli. Biochim Biophys Acta. 1976 Dec 14;455(3):889–899. doi: 10.1016/0005-2736(76)90058-4. [DOI] [PubMed] [Google Scholar]
- Honda T., Finkelstein R. A. Selection and characteristics of a Vibrio cholerae mutant lacking the A (ADP-ribosylating) portion of the cholera enterotoxin. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2052–2056. doi: 10.1073/pnas.76.4.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Cisneros L., Nalin D. R., Young C. R. Duration of infection-derived immunity to cholera. J Infect Dis. 1981 Jun;143(6):818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
- Lin Y., Means G. E., Feeney R. E. The action of proteolytic enzymes on N,N-dimethyl proteins. Basis for a microassay for proteolytic enzymes. J Biol Chem. 1969 Feb 10;244(3):789–793. [PubMed] [Google Scholar]
- Nelson E. T., Clements J. D., Finkelstein R. A. Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect Immun. 1976 Aug;14(2):527–547. doi: 10.1128/iai.14.2.527-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. R. Structural studies of immunoglobulins. Science. 1973 May 18;180(4087):713–716. doi: 10.1126/science.180.4087.713. [DOI] [PubMed] [Google Scholar]
- Robertson J. N., Vincent P., Ward M. E. The preparation and properties of gonococcal pili. J Gen Microbiol. 1977 Sep;102(1):169–177. doi: 10.1099/00221287-102-1-169. [DOI] [PubMed] [Google Scholar]
- Schneider D. R., Parker C. D. Isolation and characterization of protease-deficient mutants of vibrio cholerae. J Infect Dis. 1978 Aug;138(2):143–151. doi: 10.1093/infdis/138.2.143. [DOI] [PubMed] [Google Scholar]
- Smyth C. J., Jonsson P., Olsson E., Soderlind O., Rosengren J., Hjertén S., Wadström T. Differences in hydrophobic surface characteristics of porcine enteropathogenic Escherichia coli with or without K88 antigen as revealed by hydrophobic interaction chromatography. Infect Immun. 1978 Nov;22(2):462–472. doi: 10.1128/iai.22.2.462-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Ohman D. E., Iglewski B. H. A more sensitive plate assay for detection of protease production by Pseudomanas aeruginosa. J Clin Microbiol. 1979 Apr;9(4):538–540. doi: 10.1128/jcm.9.4.538-540.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylewska S. K., Wadström T., Hjerten S. The effect of subinhibitory concentrations of penicillin and rifampicin on bacterial cell surface hydrophobicity and on binding to pharyngeal epithelial cells. J Antimicrob Chemother. 1980 Mar;6(2):292–294. doi: 10.1093/jac/6.2.292. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Bass J. A. Role of fibronectin in the prevention of adherence of Pseudomonas aeruginosa to buccal cells. J Infect Dis. 1981 Jun;143(6):784–790. doi: 10.1093/infdis/143.6.784. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Wientjes F. B., Klaasen-Boor P. Production of K99 antigen by enterotoxigenic Escherichia coli strains of antigen groups o8, o9, o20, and o101 grown at different conditions. Infect Immun. 1980 Jan;27(1):216–221. doi: 10.1128/iai.27.1.216-221.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]